
2019
Michigan Science Advisory Workgroup
DR. JAMIE DEWITT
MR. KEVIN COX
DR. DAVID SAVITZ
HEALTH-BASED
DRINKING WATER VALUE
RECOMMENDATIONS
FOR PFAS IN MICHIGAN

i
Executive Director’s Foreword
This report accomplishes a key milestone in Michigan’s effort to
identify and reduce exposures to per- and polyfluoroalkyl
substances (PFAS) contamination. With it, we are now one step
closer to developing state drinking water standards for PFAS.
Michigan is a national leader at addressing PFAS
contamination. Through our unique, multi-agency approach,
Michigan’s PFAS Action Response Team (MPART) is
systematically identifying sources of PFAS contamination and
getting a better understanding of their occurrence throughout
our environment.
By using analytical techniques capable of finding PFAS as low
as 2 parts per trillion, we have found the presence of PFAS in the drinking water from thousands
of private residential wells near contaminated sites. We have also found PFAS in public water
supplies across the state. We tested over 1,700 supplies covering all community water supplies
plus schools and larger day cares with their own wells. We found PFAS in ten percent of the
supplies. While most of the PFAS levels were very low, three percent of the supplies have
required follow-up actions, and a few have required an alternate water source.
Unfortunately, we do not have federal drinking water standards, despite knowing they are in our
drinking water and that some PFAS have been associated with adverse health effects.
Recognizing that the USEPA is still likely several years away from providing any leadership on
PFAS drinking water standards, Michigan, like other states, was left to develop our own.
With Governor Gretchen Whitmer’s leadership, MPART formed a Science Advisory Workgroup
to navigate the science and standards from across the country to advise Michigan on drinking
water health-based values for PFAS. These health-based values will be used to inform the next
step of the drinking water rule-making process, which includes stakeholder involvement where
other factors will be considered.
I could not be more impressed with the thoughtful deliberation of our workgroup and the tireless
technical support from our staff. As the information in this report is given to EGLE for consideration
during the development of drinking water standards, we all owe them our sincere appreciation for
giving us a firm foundation on which to move forward with protecting Michiganders from
unacceptable levels of PFAS in their drinking water.
Steve Sliver,
Executive Director,
Michigan PFAS Action Response Team
ii
Michigan Science Advisory Workgroup
Dr. Jamie DeWitt
Mr. Kevin Cox
Dr. David Savitz
Agency Support Staff to the Panel
Mr. Steve Sliver, Michigan Department of Environment, Great Lakes, and Energy
Mr. Kory Groetsch, Michigan Department of Health and Human Services
Dr. Jennifer Gray, Michigan Department of Health and Human Services
Dr. Eric Wildfang, Michigan Department of Environment, Great Lakes, and Energy
Ms. Chelsea Dickerson, Michigan Department of Environment, Great Lakes, and Energy
Report developed for the Michigan PFAS Action Response Team,
Lansing, Michigan
June 27, 2019

iii
The Michigan Science Advisory Workgroup
Dr. David Savitz
Dr. David Savitz, who chairs the advisory Workgroup, is a professor of
epidemiology in the School of Public Health at Brown University. He also serves
as associate dean for research, and holds joint appointments in obstetrics and
gynecology, and pediatrics in the Alpert Medical School. His epidemiological
research has addressed a wide range of public health issues including
environmental hazards in the workplace and community, reproductive health
outcomes, and environmental influences on cancer. He has done extensive work
on health effects of nonionizing radiation, pesticides, drinking water treatment by-products, and
perfluorinated compounds. He is the author of nearly 350 papers in professional journals and editor or
author of three books. He was president of the Society for Epidemiologic Research and the Society for
Pediatric and Perinatal Epidemiologic Research, and North American regional councilor for the
International Epidemiological Association. Dr. Savitz is a member of the National Academy of Sciences
Institute of Medicine. From 2013-2017 he served as vice president for research at Brown University. He
was a member of the C8 Science Panel that conducted some of the first epidemiologic research on
PFAS in the mid-Ohio Valley and has published a number of reports related to potential health effects
of PFAS. He recently chaired the Science Panel to advise MPART on the current research related to
toxicology, epidemiology, exposure pathways, and remediation of PFAS.
Mr. Kevin Cox
Kevin Cox is a Managing Toxicologist at NSF International. Prior to his current
role, Mr. Cox was a Supervising Toxicologist supporting NSF’s drinking water
additives and dietary supplement certification programs. As an expert in human
health risk assessment, Mr. Cox has authored numerous chemical risk
assessments evaluating exposure from unregulated drinking water contaminants,
dietary supplement ingredients, toy product materials, and pool and spa treatment
chemicals. Specific to PFAS, Mr. Cox has conducted a state-of-the-science analysis of published PFAS
risk assessments in support of NSF International drinking water programs. This analysis was recently
presented to Michigan water management professionals. Mr. Cox received his B.S. in biochemistry and
history from the University of Michigan and his MPH in Environmental Health Sciences - Toxicology
from the University of Michigan School of Public Health. He is currently an Associate Member of the
Society of Toxicology. Mr. Cox also holds a J.D. from the University of Michigan Law School and is a
member of the Michigan Bar Association.
Dr. Jamie DeWitt
Dr. Jamie DeWitt is an associate professor in the Department of Pharmacology and
Toxicology of the Brody School of Medicine at East Carolina University. Her
laboratory’s research program explores relationships between biological organisms
and their responses after exposure to environmental contaminants, with a specific
focus on the immune system and its interactions with the nervous system during
development and adulthood. The research program particularly focuses on
emerging aquatic contaminants, especially PFAS. With respect to PFAS, DeWitt has published 13
primary research articles, six review articles, two book chapters, and edited a book on PFAS toxicity.
She has served as an external reviewer for the United States Environmental Protection Agency
(USEPA) health effects assessment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate
(PFOS), the United States National Toxicology Program’s immune effects assessment of PFOA and
iv
PFOS, the United States Agency for Toxic Substances and Disease Registry toxicological profile for
PFASs, and was a member of the International Agency for Research on Cancer working group for the
assessment of the carcinogenicity of PFOA. Her laboratory currently assesses the immunotoxicity of
emerging PFAS that have been designed to replace those that have been phased out of production and
that are of concern in North Carolina. She double-majored in environmental science and biology for her
bachelor’s degree from Michigan State University and has doctoral degrees in environmental science
and neural science from Indiana University-Bloomington. She completed postdoctoral training in
ecotoxicology at Indiana University-Bloomington and in immunotoxicology at the USEPA in partnership
with the University of North Carolina at Chapel Hill.

1
Table of Contents
Executive Director’s Foreword ..................................................................................................... i
The Michigan Science Advisory Workgroup ............................................................................... iii
Executive Summary ................................................................................................................... 2
Approach ................................................................................................................................... 5
Workgroup Interpretation of the Charge .................................................................................. 5
Challenges and Limitations ..................................................................................................... 6
Process ...................................................................................................................................... 7
Selection of Toxicity Values ................................................................................................... 7
Uncertainty Factors ................................................................................................................ 7
Relative Source Contribution .................................................................................................. 8
Drinking Water Health-Based Value Derivation ...................................................................... 8
Confidence Statement ............................................................................................................ 9
PFAS Chemical Summary Sheets.............................................................................................10
Chemical Summary for PFNA ............................................................................................10
Chemical Summary for PFOA ............................................................................................12
Chemical Summary for PFHxA ..........................................................................................14
Chemical Summary for PFOS ............................................................................................16
Chemical Summary for PFHxS ..........................................................................................18
Chemical Summary for PFBS ............................................................................................20
Chemical Summary for GenX .............................................................................................22
Rationale for Individual HBVs ....................................................................................................25
Summary of Conclusions ..........................................................................................................26
Summary Table of Drinking Water HBVs ...............................................................................26
Figure 1. ............................................................................................................................27
Concluding Remarks .................................................................................................................27
References ...............................................................................................................................29
Appendix A: Acronym List ......................................................................................................33
Appendix B: MPART Motion for Creation of Science Advisory Workgroup, April 4, 2019 ......34
Appendix C: USEPA Method 537.1 Analyte List ....................................................................35
Appendix D: Timeline for the Science Advisory Workgroup’s Development of Drinking Water
HBVs .....................................................................................................................................36
Appendix E: Timeline of the Maximum Contaminant Level Development Process .................37

2
Executive Summary
Background: The Michigan PFAS Action Response Team (MPART), is a unique, multi-agency
proactive approach for coordinating state resources to address per- and polyfluoroalkyl
substances (PFAS) contamination. Agencies responsible for environmental protection, public
health, natural resources, agriculture, military installations, commercial airports, and fire
departments work together to ensure the most efficient and effective response. The work done
by MPART on drinking water supports the development of standards now that we have key
information, including:
• PFAS have been discovered in drinking water during investigations of contaminated sites
and a survey of all of Michigan’s public water supplies. Public health responses, such as
the provision of alternate water (e.g., point of use filters) have been necessary for
thousands of Michiganders based on the strength of the source, location, and the
concentrations found.
• The MPART Science Advisory Panel report issued in December 2018 indicated that
observational epidemiology literature supports the need for drinking water values below
the United States Environmental Protection Agency (USEPA) Lifetime Health Advisory
(LHA) level of 70 ppt PFOS and PFOA, individually or in combination, and included a
recommendation for establishing state drinking water standards for PFAS.
• The Michigan Department of Health and Human Services (MDHHS)-led MPART Human
Health Workgroup developed public health drinking water screening levels for five
individual PFAS in February 2019. Those screening levels will prompt further evaluation
and public health consultations at numerous public water supplies and residences across
the state including where detectable levels of PFOS and/or PFOA are below the USEPA
LHA.
On March 26, 2019, Governor Gretchen Whitmer announced that Michigan was establishing
enforceable state drinking water standards for PFAS. These standards, otherwise known as
Maximum Contaminant Levels (MCLs), under the federal Safe Drinking Water Act have
traditionally been established first by the USEPA and then adopted by the states. At this time,
however, the USEPA has not initiated its process for establishing PFAS MCLs, and its process
could take five or more years to complete. Michigan chose not to wait any longer for federal action.
Governor Whitmer called on MPART to form a Science Advisory Workgroup (Workgroup) to
review the existing and proposed PFAS standards from across the country and develop health-
based values (HBVs) to inform the initial phase of the rulemaking process for establishing state
drinking water standards. The workgroup was given until July 1, 2019 to develop the HBVs. On
April 4, 2019, MPART approved a motion to create the Workgroup. The Charge from MPART to
the Workgroup is included in Appendix B. The members of the Workgroup were announced on
April 11, 2019. The Workgroup was supported by MPART staff.

3
The Workgroup members are experts in the fields of epidemiology, toxicology, and risk
assessment. The composition of the Workgroup matches the typical fields of evaluation for HBV
developments. Dr. Jamie DeWitt provided the strong toxicological expertise and up-to-date
knowledge on PFAS toxicology as HBVs typically use laboratory animal toxicity studies.
Epidemiological information supports the laboratory animal data, and Dr. David Savitz provided
his epidemiological expertise in selection of health endpoints and relevance to humans. Tying
both toxicology and epidemiology together are risk assessment practices, and Mr. Kevin Cox
provided the expertise in that field. Taken together, this Workgroup was able to knowledgably
speak on the current state of PFAS health research and provide the scientific expertise needed
to efficiently develop HBVs on the requested timeline.
The evaluation and deliberations of the Workgroup occurred over a very limited timeframe
(Appendix D), which required frequent interaction. Much of that interaction occurred during 7 web
conferences between April 19 and May 29, 2019, culminating in an in-person meeting the weekend
of June 1-2, 2019. The Workgroup’s final conclusions were presented to MPART on June 27, 2019.
Conclusions: The Workgroup undertook a methodical approach to evaluate existing and
proposed standards from across the country for the 18 PFAS analytes considered under USEPA
Method 537.1 (Appendix C). They focused on those PFAS that they determined had enough peer
reviewed studies on which to base their conclusions. What they considered, and the logic behind
their approach, has been carefully documented in individual chemical summaries for each
compound that has a derived HBV in the following table:
Summary Table of Drinking Water Health-Based Values
Specific
PFAS
Drinking Water Health-
based Value
Chemical Abstract
Services Registry
Number (CASRN)
PFNA
6 ng/L (ppt)
375-95-1
PFOA
8 ng/L (ppt)
335-67-1
PFHxA
400,000 ng/L (ppt)
307-24-4
PFOS
16 ng/L (ppt)
1763-23-1
PFHxS
51 ng/L (ppt)
355-46-4
PFBS
420 ng/L (ppt)
375-73-5
GenX
370 ng/L (ppt)
13252-13-6
The Workgroup also recommended MPART and water supply operators screen analytical results
for other long-chain PFAS (eight carbons and above for carboxylates and six carbons and above
for sulfonates) included in USEPA Method 537.1 at the lowest concentration proposed for any of
the compounds, which is 6 ppt. Based on the similarity in toxicity for the long-chain PFAS, the
Workgroup recommends use of the HBV for PFNA (6 ng/L [ppt]) as a screening level for all other
long-chain PFAS included on the USEPA Method 537.1 analyte list for which the Workgroup did
not develop an individual HBV. Those other long-chain PFAS included in USEPA Method 537.1
are: NEtFOSAA (CASRN: 2991-50-6); NMeFOSAA (CASRN: 2355-31-9); PFDA (CASRN: 335-
76-2); PFDoA (CASRN: 307-55-1); PFTA (CASRN: 376-06-7); PFTrDA (CASRN: 72629-94-8);
and PFUnA (CASRN: 2058-94-8). While there is not enough information available at this time to
support HBVs and drinking water standards for them, these compounds are expected to produce
similar health effects. Additional monitoring, research for potential sources, notification of the
public, and efforts to reduce exposure are warranted.
4
The Workgroup recognizes that their conclusions in some cases deviate modestly from those of
other organizations. Evolving science and professional judgement can account for the variation.
The variation is not substantial, however, and the values are trending lower nationally over time.

5
Approach
Workgroup Interpretation of the Charge
The Workgroup was conscience of the importance and responsibility placed upon its efforts to
identify public health toxicity values for certain PFAS as described within the Charge. Prior to
initiating its efforts, the Workgroup sought and received clarification on the scope of the Charge.
Given the relatively short timeframe for which to accomplish the tasks set forth within Charge, the
Workgroup confirmed that the focus of the effort was to utilize the existing and proposed national-
and state-derived PFAS assessments to inform its decision-making process as opposed to
conducting a full systematic review of the available scientific literature on PFAS.
Additionally, as one of the outputs of the Charge is to inform State of Michigan on drinking water
health-based values for PFAS, it was important to understand if the State of Michigan had any
paradigms in place that the Workgroup must follow when deriving drinking water health-based
values. The response received from the State of Michigan indicated that the Workgroup was only
limited to applying a scientifically defensible approach as described within the Charge. With these
issues clarified, the Workgroup approached the tasks set forth in the charge in the following
manner:
1) Initially, PFAS analytes were identified within USEPA Method 537.1 for which published
or externally peer reviewed PFAS drinking water criteria or reference doses (RfDs) existed
and the derivation of such values was done in a scientifically defensible manner. This
approach resulted in the selection of PFOA, PFOS, PFHxS, PFHxA, PFBS, PFNA and
GenX as PFAS analytes for which the Workgroup would then develop individual public
health toxicity values. The remaining PFAS values within USEPA Method 537.1 were later
considered as to whether a class-based or group-based public health toxicity value could
be applied.
2) For each of the selected PFAS analytes, the Workgroup evaluated the identified points of
departure (defined as the point on a toxicological dose-response curve corresponding to
an estimated low effect level or no effect level) and rationale from published risk
assessments and assessed the underlying key studies that served as the basis for the
published values. From this review, the merits of each available point of departure was
discussed among the Workgroup and critical studies and points of departures for each of
the seven identified PFAS analytes were identified to form the basis of public health toxicity
values described further herein.
3) With critical studies and points of departure identified for each individual PFAS, the
Workgroup then identified appropriate uncertainty factors to derive public health toxicity
values. From these public health toxicity values, the Workgroup recommended specific
drinking water exposure paradigms, accounting for sensitive sub-populations, and applied
selected relative source contribution factors to derive the drinking water health-based
values described further herein.
4) Lastly, consideration was given to the remaining PFAS analytes from USEPA Method
537.1 that were not selected for the development of individual criteria as to whether a
class-based or grouping-based evaluation approach would be appropriate. As described
6
below, the Workgroup concluded that a screening level approach was valid to assess
longer-chain PFAS based on the lowest derived drinking water health-based values.
Based on guidance from the Director of EGLE’s Drinking Water and Environmental Health
Division, PFAS chemical summary sheets were used to capture the necessary information for the
MCL rulemaking process. The Workgroup and MPART staff used this format to provide maximum
transparency on the decisions and rationale for drinking water health-based value development
for each PFAS.
The chemical summary sheets describe:
• The critical study or studies, point of departure from each study, and conversion to a
human equivalent dose;
• Uncertainty factors and a calculated toxicity value;
• Exposure parameters, and methodology for calculation of a drinking water health-based
value.
Challenges and Limitations
The premises for the Workgroup’s efforts to provide evidence-based conclusions for informing the
regulation of PFAS in drinking water are compelling. Policy needs to provide clarity on what levels
of specific chemicals are believed to be protective of public health and develop a mechanism to
monitor and mitigate pollutants such as PFAS where needed. The Workgroup identified and
made optimal use of the scientific evidence that is available to provide guidance, drawing on its
knowledge of research methods and quantitative risk assessment. Furthermore, the Workgroup
approached the issue free of bias, and as a panel, has a wide range of expertise and familiarity
with the research on PFAS. However, the nature of this process is inherently subject to
uncertainty and other equally qualified experts presented with the same scientific data the
Workgroup drew upon might well make somewhat different conclusions. A number of other
organizations have been through a similar exercise in providing guidance on acceptable drinking
water contaminant levels, and while there are not extreme differences, there is not complete
convergence either. As described in some detail below, a series of inputs were needed to derive
the Workgroup’s estimates and make that sequence of decisions as transparent as possible for
those who wish to compare these conclusions to those made by other agencies. Like all the
others, they are based exclusively on toxicology studies given the ability to quantify exposure-
response relationships with great precision, but there is a loss of certainty in applying these
estimates to free-living human populations. In most cases, there is epidemiologic evidence
pertaining to the same health endpoints used in toxicology, and where there is such convergent
evidence (e.g., immune function, development), confidence in the applicability of the experimental
studies to human populations is enhanced. Finally, it should be noted that the scientific evidence
on PFAS is expanding rapidly and that with new studies, the guidelines may well need to be
revised. While it would be inefficient to do so frequently, on some periodic basis of several years,
it would be useful to repeat the process that generated this report to determine where changes
may be needed.
7
Process
Selection of Toxicity Values
Adverse health effects reported following exposure to PFAS in laboratory animal models and
epidemiological studies have been summarized in myriad peer-reviewed and publicly available
documents, including those generated by other state agencies. Most recently, the Agency for
Toxic Substances and Disease Registry (ATSDR), compiled a toxicological profile for 14 PFAS
that comprehensively summarizes evidence from publicly available published studies (ATSDR,
2018). This, and other summary documents, as well as the published studies themselves, were
relied on to determine points of departure, as well as the toxicity values that protect the most
sensitive populations and reflect a level that is unlikely to lead to adverse health effects if those
sensitive populations are exposed over a lifetime or during a sensitive period (i.e., during
development). The toxicity values are therefore designed to be protective of all exposed
populations. For all of the PFAS examined, points of departure were selected from studies with
laboratory animal models. This approach does not negate findings associated with
epidemiological studies, but reflects that humans experience uncontrolled and imperfectly
documented rather than controlled, precisely measured exposures. Additionally, these points of
departure reflect adverse health effects that occur at low doses and that are supported by the
weight-of-evidence across endpoints and between findings in humans and laboratory animal
models. Therefore, the process to select points of departure used the available scientific evidence
to identify an adverse health effect that occurred at a low dose, was supported by findings in other
studies, was relevant to humans, and would be protective of sensitive populations.
Uncertainty Factors
In deriving the toxicity values for PFAS, the selected points of departure are divided by uncertainty
factors. Uncertainty factors are applied in order to account for:
1. Variation in susceptibility among the human population (intraspecies uncertainty);
2. Uncertainty in extrapolating animal data to humans (interspecies uncertainty);
3. Uncertainty in extrapolating from data obtained from a study with a less-than-lifetime
exposure (subchronic to chronic uncertainty);
4. Uncertainty in extrapolating from a lowest observed adverse effect level (LOAEL) as
opposed to a no observed adverse effect level (NOAEL); and
5. Uncertainty associated with an incomplete toxicity database. Uncertainty factors assigned
for each of these five categories are typically 1x, 3x (10
0.5
x), or 10x with the default value
being 10x, which represents greater uncertainty.
For both interspecies and intraspecies uncertainty factors, the variability in response to a toxicant
may result from differences in toxicokinetics and/or toxicodynamics. Toxicokinetics refers to the
absorption, distribution, biotransformation and excretion of the toxicant following exposure.
Toxicodynamics refers to the molecular, biochemical and physiological effects of the toxicant or
its metabolites leading to the toxic response. Therefore, the interspecies and intraspecies
uncertainty factors are divided into subparts representing the toxicokinetic factor and the
toxicodynamic factor. In evaluating the interspecies uncertainty for the selected PFAS, in each
8
case the toxicokinetic subfactor was able to be reduced to 1x on account of adjustments based
on serum half-lives or allometric scaling. Due to lack of data to depart from the default the
toxicodynamic subfactor 3x (10
0.5
x), the resulting interspecies uncertainty factor is 3x (10
0.5
x).
When considering the subchronic to chronic uncertainty, the relevant consideration is whether the
selected point of departure may differ if the duration of exposure were to be increased. For PFAS,
a weight of evidence approach was used to assess the subchronic to chronic uncertainty factor,
including, but not limited to, duration of the key study, potential impact of duration on the selected
point of departure, as well as availability of chronic repeat-dose toxicity data.
For the NOAEL to LOAEL uncertainty factor, use of a NOAEL (or lower confidence limit on the
benchmark dose [BMDL]) allows for an uncertainty factor of 1x. If the point of departure is based
on a LOAEL, the uncertainty factor is either 3x (10
0.5
x) or 10x depending on the severity and/or
reversibility of the critical effect.
The database uncertainty factor is based on the ability of the existing data to support a scientific
judgment of the likely critical effect from exposure to the compound. In assessing the database
completeness, the types of toxicity data (e.g., human, animal, mode of action) as well as data
gaps that may have improved the derived risk values should be emphasized. This approach
should take into consideration issues such as the types of endpoints evaluated, life-stages
evaluated, duration, timing, route of exposure, and the potential for latent effects and/or
reversibility of effects (USEPA, 2002). For the selected PFAS, each database was unique;
however, common concerns were lack of appropriate characterization of immune, endocrine or
neurodevelopmental effects.
Relative Source Contribution
Relative source contribution (RSC) is the percentage of a person’s exposure to a chemical that
comes from drinking water. For example, an RSC of 20 percent assumes that the other 80
percent of a person’s exposure to a chemical comes from non-drinking water sources. The
USEPA (2000) provides guidance on the selection of an RSC value using an exposure decision
tree that takes into account specific populations of concern, whether these populations are
experiencing exposure from multiple sources, and whether levels of exposure or other
circumstances make apportionment of the toxicity value or POD/UF desirable. The most
conservative RSC is established at 20 percent, and the RSC can reach a ceiling of 80 percent
as more information is available about exposure pathways and the source of exposure.
Drinking Water Health-Based Value Derivation
The traditional risk assessment approach using simple equations based on body weight, water
intake rate and RSC to calculate drinking water HBVs is not adequate to address the
bioaccumulative nature and known or presumed developmental toxicity of PFAS. These
traditional equations do not consider the PFAS body-burden at birth or any transfer of maternal
PFAS through breastmilk. To better address these concerns, and to also account for higher early-
life intake rates, the Goeden et al. (2019) simple one-compartment toxicokinetic model was used
where the data were available for the individual PFAS. The resulting drinking water HBVs are
considered protective for an infant exclusively breast-fed for 12 months, followed by drinking
contaminated water through life. Additionally, these drinking water HBVs also protective for
formula-fed infants. Where data were not available to derive drinking water HBVs using the model,
traditional equations were used.
9
Confidence Statement
Following USEPA guidance (2002), risk assessments may contain a narrative description of the
overall confidence in the derived health-effects based values. Confidence in the risk assessment
would be low if there is a high degree of scientific uncertainty and would be high if there is a low
degree of scientific uncertainty. Major elements of scientific uncertainty may be considered to
include, but not limited to, the following; database completeness, quality of key study(ies), severity
and relevance of the critical effect, quality of the dose-response analysis and consideration of
sensitive subpopulations. (NRC, 2009; Beck et al., 2016).
For the selected PFAS for which quantitative values were derived there remains significant
scientific uncertainty. Health outcomes due to PFAS exposure that warrant additional study
include, but are not limited to, endocrine disruption, immunological and neurodevelopmental
effects as well as cancer. Further information is needed on the mode of action as well as the
cumulative risk of exposure to multiple PFAS. Overall, the present evaluation of the selected
PFAS is based on sound science and current practices in risk assessment; however, the
Workgroup recognizes that the science of PFAS is constantly evolving and new information may
come to light that requires a re-evaluation of the drinking water HBVs established herein.
10
PFAS Chemical Summary Sheets
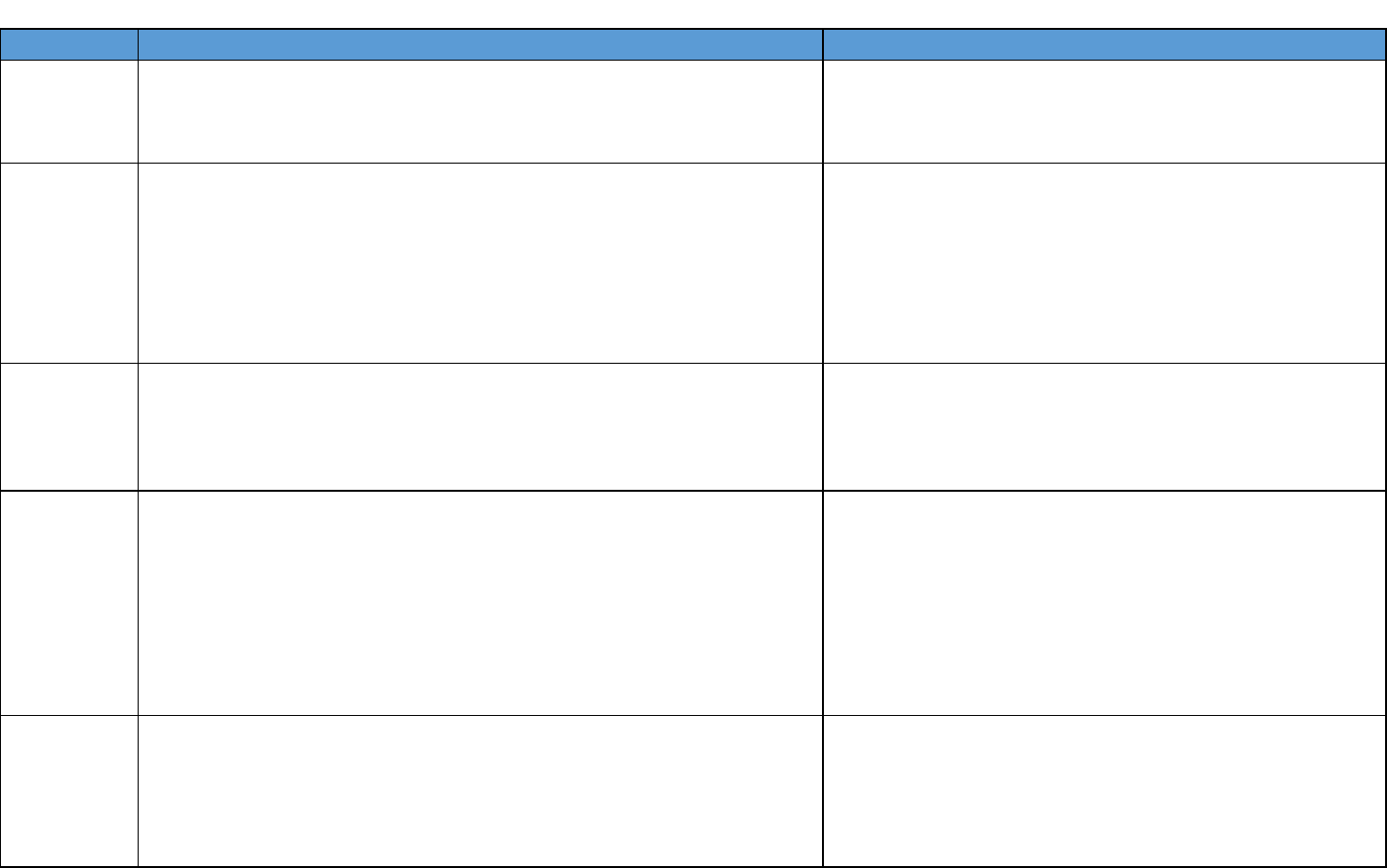
Chemical Summary for PFNA
Decision Point
Rationale/justification
Critical
study
Das KP, Grey BE, Rosen MB, et al. 2015. Developmental toxicity
of perfluorononanoic acid in mice. Reproductive Toxicology 51:133-
144.
The Workgroup reviewed the available evaluations and
focused on the assessments by ATSDR and New Jersey.
Das et al. (2015) was selected by both ATSDR (2018)
and NJDEP (2015).
Description
of the critical
study
Timed-pregnant CD-1 mice were administered 0, 1, 3, 5 or 10 mg/kg
PFNA by daily oral gavage from gestational day (GD) 1 to 17. Maternal
toxicity and reproductive outcomes were investigated. Postnatal
toxicity, liver gene expression and developmental effects were
evaluated in mouse offspring.
Body weight endpoints – Decreased body weight gain in mouse pups
Developmental endpoints – Delayed eye opening, preputial separation,
and vaginal opening in mouse pups
The Workgroup reviewed the health endpoints
investigated in Das et al. (2015) and identified the
developmental endpoints as more relevant than liver
endpoints.
Point of
Departure
(POD)
A NOAEL of 1 mg/kg/day was identified for developmental effects. The
average serum concentration for NOAEL (1 mg/kg/day) was estimated
(6.8 mg/L) in dams using an empirical clearance model (Wambaugh et
al., 2013). The estimated time-weighted average serum concentration
corresponding to the NOAEL was 6.8 mg/L.
The Workgroup decided that serum-based points of
departure were appropriate for PFAS.
Human
equivalent
dose (HED)
The time-weighted average serum concentration of 6.8 mg/L was
converted to the HED using the below equation.
NOAEL
HED
= (TWA serum x k
e
x V
d
) = 0.000665 mg/kg/day
Ke = 0.000489165 (4.8 x 10
-4
) based on a human serum half-life of
1417 days (calculated from Zhang et al. [2013] as described above)
Vd = 0.2 L/kg (ATSDR [2018]; Ohmori et al. [2003])
The Workgroup discussed the human serum half-lives
available from Zhang et al. (2013), which were
an arithmetic mean of 2.5 years (913 days) for 50 year old
or younger females and 4.3 years (1570 days)
for females older than 50 years old and all males. An
average of 3.9 years (1417 days) was calculated based on
those averages. The Workgroup selected the calculated
average as it would better represent the entire
population.
Uncertainty
factors
A total uncertainty factor of 300:
• 1 for LOAEL to NOAEL
• 10 for human variability
• 3 (10
0.5
) for animal to human variability
• 1 for subchronic to chronic
• 10 for database deficiencies was used.
The Workgroup discussed the uncertainty factors selected
by ATSDR (2018) and agreed that those selected were
appropriate.
11
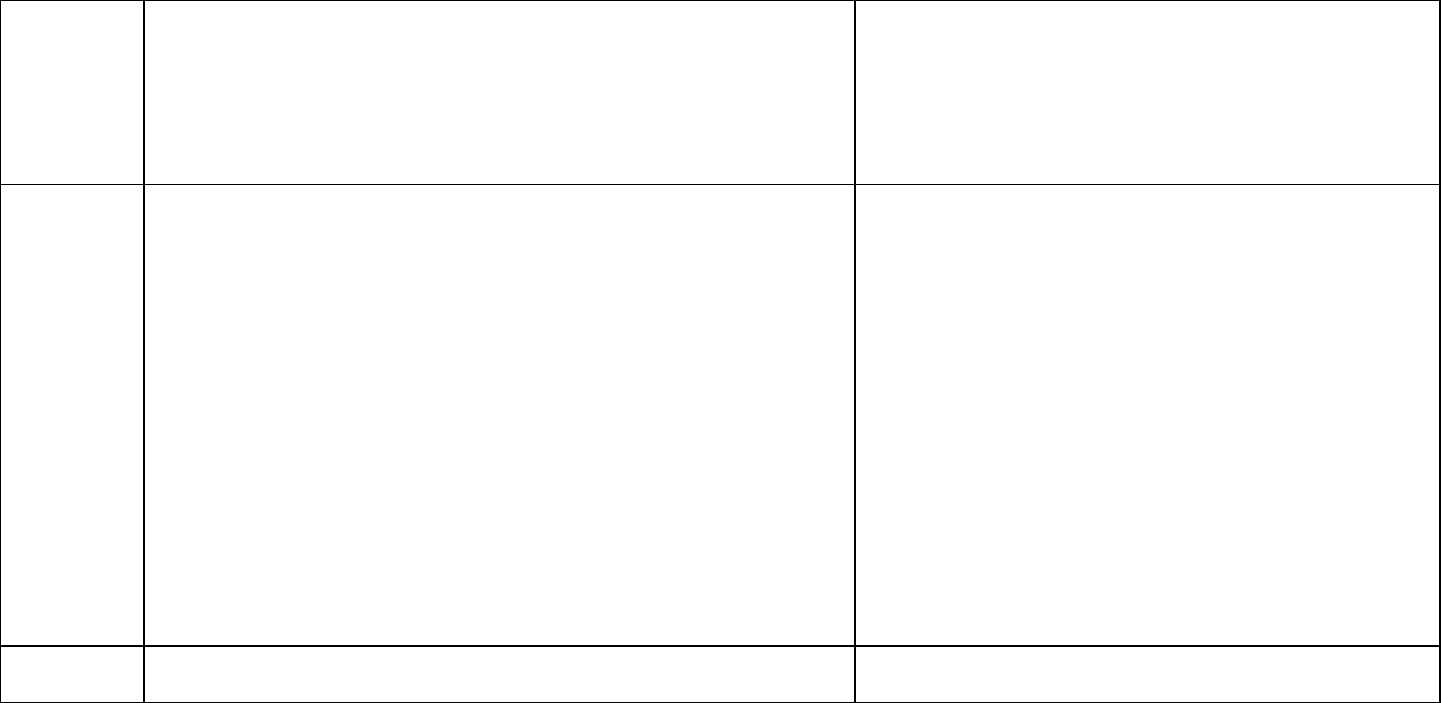
Toxicity
value
2.2 ng/kg/day (2.2 x 10
-6
mg/kg/day) which corresponds to a serum
concentration of 0.023 mg/L
Serum levels used in development of these toxicity levels are not
meant to indicate a level where health effects are likely. These serum
levels are calculated to be at a point where no or minimal risk exists for
people drinking water with a certain PFAS.
Human equivalent dose or serum level divided by the total
uncertainty factors = toxicity value
Exposure
parameters
for drinking
water
screening
HBVs
Breast-fed infant, which is also protective of a formula-fed infant
Placental transfer of 69% (MDHHS 2019)
Breastmilk transfer of 3.2% (MDHHS 2019)
Half-life = 1417 days (3.9 years) (calculated from Zhang et al. [2013] as
described above)
Volume of distribution = 0.2 L/kg (ATSDR [2018]; Ohmori et al. [2003])
95
th
percentile drinking water intake, consumers only, from birth to more
than 21 years old (Goeden et al. [2019])
Upper percentile (mean plus two standard deviations) breast milk
intake rate (Goeden et al. [2019])
Time-weighted average water ingestion rate from birth to 30-35 years
of age (to calculate maternal serum concentration at delivery)
(Goeden et al. [2019])
Relative Source Contribution of 50% (0.5)
Based on NHANES 95
th
percentiles for 3-11 (2013-2014) and over 12
years old (2015-2016) participants (CDC 2019)
The Workgroup discussed the Goeden et al. (2019) model
which considered full life stage exposure, from fetal
exposure, to infant exposure through breastfeeding, and
into adulthood. While the model was also developed for a
formula-fed infant, the breastfed infant scenario is
protective of a formula-fed infant. The Workgroup selected
this model for developing drinking water HBVs when the
needed inputs were available.
Drinking
water HBV
6 ng/L (ppt)
Numeric HBV derived and justified using the above
information
12
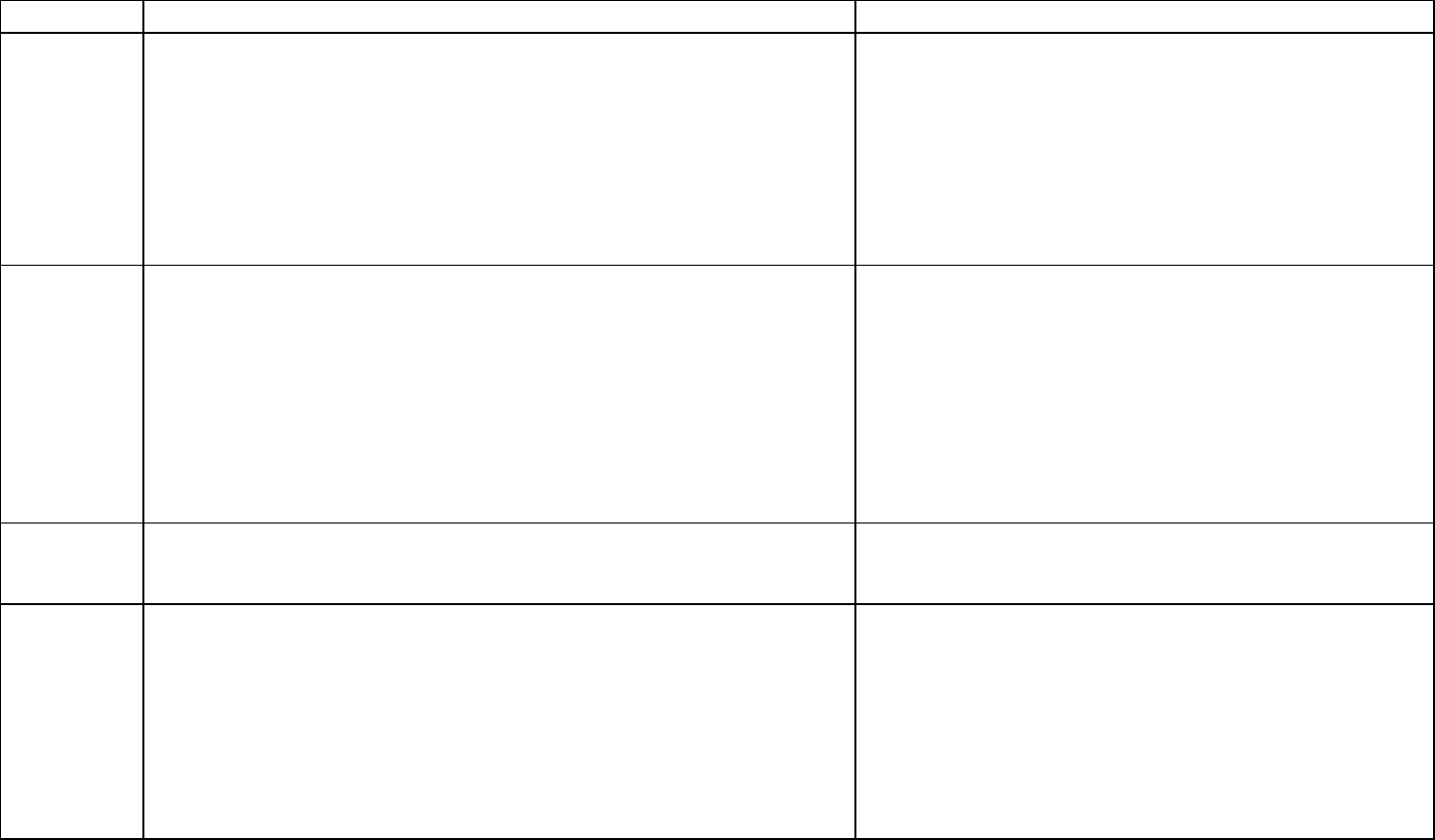
Chemical Summary for PFOA
Decision point
Rationale/justification
Critical
study
Onishchenko N, Fischer C, Wan Ibrahim WN, Negri S, Spulber S,
Cottica D, Ceccatelli S. 2011. Prenatal exposure to PFOS or PFOA
alters motor function in mice in a sex-related manner. Neurotox. Res.
19(3):452-61.
Koskela A, Finnilä MA, Korkalainen M, Spulber S, Koponen J, Håkanss
on H, Tuukkanen J, Viluksela M. 2016. Effects of developmental
exposure to perfluorooctanoic acid (PFOA) on long bone morphology
and bone cell differentiation. Toxicol. Appl. Pharmacol. 301:14-21.
The Workgroup reviewed the available evaluation and
selected the ATSDR (2018) critical studies. The
Workgroup concluded that the ATSDR
position was defensible with respect to range and
sensitivity of health endpoints identified and considered in
ATSDR (2018).
Description
of the critical
study
Onishchenko et al.: Pregnant C57BL/6 mice were exposed to 0 or 0.3
mg PFOA/kg/day throughout pregnancy. The critical effects considered
were Neurobehavioral effects (decreased number of inactive periods,
altered novelty induced activity) at 5-8 weeks of age.
Koskela et al.: Pregnant C57BL/6 mice were exposed to PFOA mixed
with food at the dose of 0 or 0.3 mg PFOA/kg/day throughout
pregnancy. Group of five offspring (female) were sacrificed at either 13
or 17 months of age. The critical effects considered were skeletal
alteration such as bone morphology and bone cell differentiation in the
femurs and tibias.
The Workgroup selected these
developmental delays as most appropriate health
endpoint as the mammary gland effects may represent a
delay that may not be considered adverse. However, the
mammary gland effects may be representative of
endocrine effects at doses below the selected POD.
Point of
Departure
The average serum concentration was estimated in the mice (8.29
mg/L) using a three-compartment pharmacokinetic model (Wambaugh
et al. 2013) using animal species-, strain-, sex-specific parameters.
The Workgroup decided that serum-based points of
departure were appropriate for PFAS.
Human
equivalent
dose
The time-weighted average serum concentration of 8.29 mg/L was
converted to the HED using the below equation.
LOAEL
HED
= (TWA serum x k
e
x V
d
) = 0.001163 mg/kg/day
Ke = 0.000825175 (8.2 x 10
-4
) based on a human serum half-life of 840
days (Bartell et al. 2010)
Vd = 0.17 L/kg (Thompson et al. 2010)
The Workgroup selected the PFOA serum half-life of 840
days (2.3 years) as more relevant for exposure to the
general population as this half-life corresponds to data
from Bartell et al. (2010) in which 200 individuals (100
men, 100 women) were exposed by drinking PFOA-
contaminated water.
The Workgroup selected the volume of distribution based
on human data, when available.
13
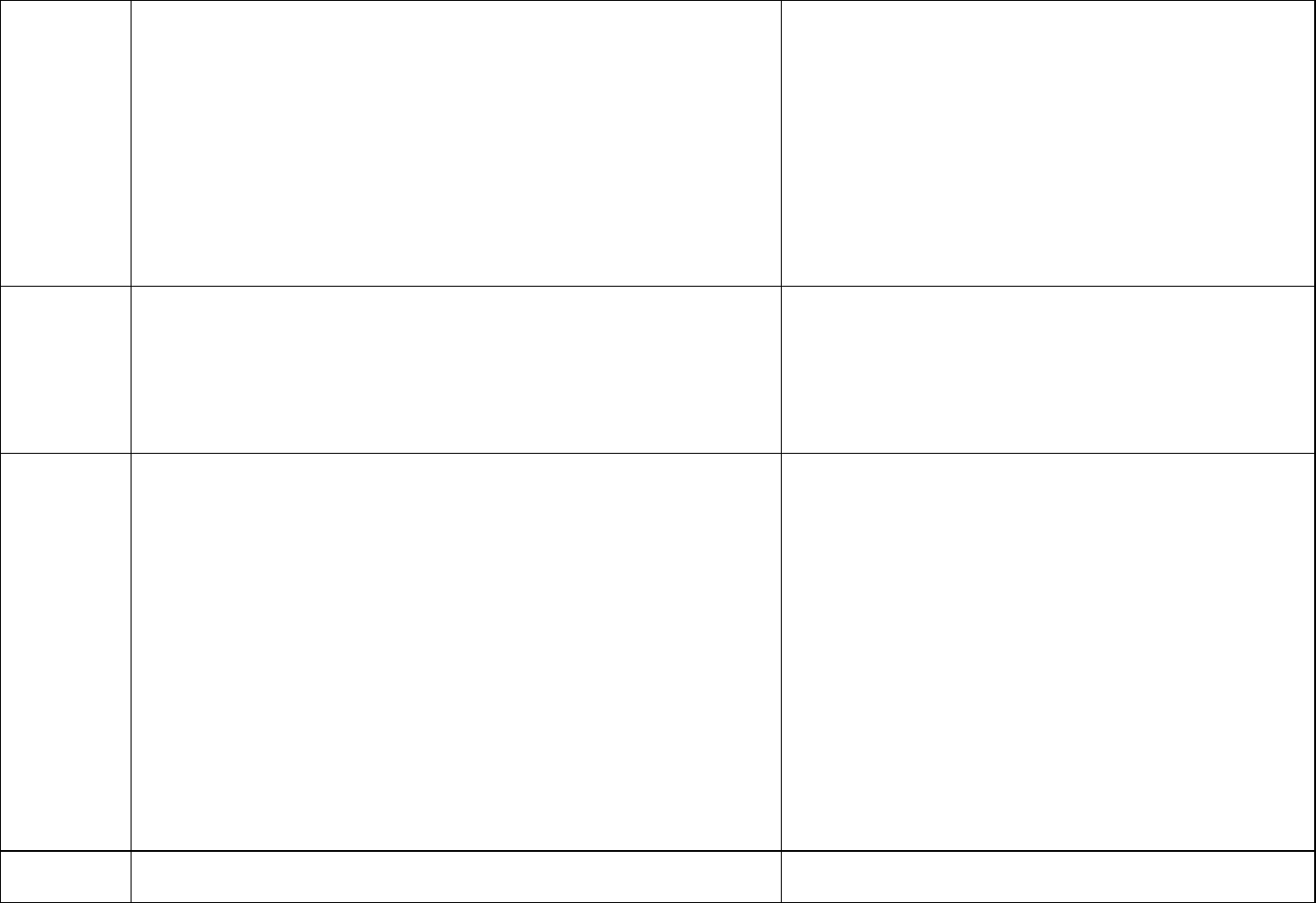
Uncertainty
factors
A total uncertainty factor of 300:
• 3 (10
0.5
) for LOAEL to NOAEL
• 10 for human variability
• 3 (10
0.5
) for animal to human variability
• 1 for subchronic to chronic
• 3 (10
0.5
) for database deficiencies (endocrine effects)
The Workgroup discussed the use of an uncertainty factor
of 3 for use of a LOAEL. They noted that a NOAEL for
immune effects was similar to the LOAEL selected and
that the selected LOAEL represented less severe effects.
The Workgroup concluded that use of the 3 (10
0.5
) would
be sufficiently protective.
The Workgroup added a database uncertainty factor of
3 (10
0.5
) for deficiencies the database regarding endocrine
effects. The Workgroup noted that the mammary gland
effects may signal a concern for other low dose endocrine
effects.
Toxicity
value
3.9 ng/kg/day (3.9 x 10
-6
mg/kg/day) which corresponds to a serum
concentration of 0.028 mg/L
Serum levels used in development of these toxicity levels are not
meant to indicate a level where health effects are likely. These serum
levels are calculated to be at a point where no or minimal risk exists for
people drinking water with a certain PFAS.
Human equivalent dose or serum level divided by the total
uncertainty factors = toxicity value
Exposure
parameters
for drinking
water HBVs
Breast-fed infant, which is also protective of a formula-fed infant
Placental transfer of 87% (MDH 2017)
Breastmilk transfer of 5.2% (MDH 2017)
Human Serum half-life of 840 days (Bartell et al. 2010)
Volume of distribution of 0.17 L/kg (Thompson et al. [2010])
95
th
percentile drinking water intake, consumers only, from birth to
more than 21 years old (Goeden et al. [2019])
Upper percentile (mean plus two standard deviations) breast milk
intake rate (Goeden et al. [2019])
Time-weighted average water ingestion rate from birth to 30-35 years
of age (to calculate maternal serum concentration at delivery) (Goeden
et al. [2019])
Relative Source Contribution of 50% (0.5)
Based on NHANES 95
th
percentiles for 3-11 (2013-2014) and over 12
years old (2015-2016) participants (CDC 2019)
The Workgroup discussed the Goeden et al. (2019) model
which considered full life stage exposure, from fetal
exposure, to infant exposure through breastfeeding, and
into adulthood. While the model was also developed for a
formula-fed infant, the breastfed infant scenario is
protective of a formula-fed infant. The Workgroup selected
this model for developing drinking water HBVs when the
needed inputs were available.
Drinking
water HBV
8 ng/L (ppt)
Numeric HBV derived and justified using the above
information
14
Chemical Summary for PFHxA
Decision point
Rationale/justification
Critical
study
Klaunig, J.E., Shinohara, M., Iwai, H., Chengelis, C.P., Kirkpatrick, J.B.,
Wang, Z., Bruner, R.H., 2015. Evaluation of the chronic toxicity and
carcinogenicity of perfluorohexanoic acid (PFHxA) in Sprague-Dawley
rats. Toxicol. Pathol. 43 (2), 209–220.
The Workgroup reviewed the Luz et al. (2019) compiled
information and development of a toxicity value. The
Workgroup was in agreement with Luz et al. (2019) on
selection of the chronic study (Klaunig et al. 2015) for
toxicity value development.
Description
of the critical
study
PFHxA was administered to male and female Crl:CD rats (n=60-
70/sex/dose) via daily oral gavage for up to 104 weeks. Males: 0, 2.5,
15, and 100 mg/kg/day. Females: 0, 5, 30, and 200 mg/kg/day.
Functional observational battery, locomotor activity, ophthalmic,
hematology, serum chemistry, and tissue and organ histopathology
endpoints were evaluated.
The Workgroup also considered the developmental effects
observed in Loveless et al. (2009) one generation
reproductive assay. Pup body weight was significantly
reduced in the 500 mg/kg/day, resulting in NOAEL of 100
mg/kg/day. Data were not available for Benchmark Dose
Modeling for further evaluation.
Point of
Departure
Critical effect renal tubular degeneration and renal papillary necrosis in
female rats – BMDL
10
90.4 mg/kg/day (Luz et al., 2019).
The Workgroup noted that the Benchmark Dose approach
is preferred over the use of a NOAEL/LOAEL.
Human
equivalent
dose
Therefore, the BMD was adjusted by (80kg/0.45 kg)
¼
= 3.65. The
resulting POD
HED
(90.4 mg/kg/day divided by 3.65) = 24.8 mg/kg/day.
(Luz et al., 2019).
The Workgroup discussed the description of the
Benchmark Dose modeling conducted by Luz et al. (2019)
and concluded the modeling was adequate for use. The
Workgroup did not conduct their own Benchmark Dose
modeling.
The Workgroup took into consideration the available
serum half-life data presented in Russell et al. (2013) and
concluded that, unlike most PFAS, allometric scaling could
be supported.
Uncertainty
factors
Total uncertainty factor of 300:
• 1 for LOAEL to NOAEL
• 10 for human variability
• 3 (10
0.5
) for animal to human variability
• 1 for subchronic to chronic
• 10 for database deficiencies – lack of additional chronic toxicity
studies and no additional developmental data in a second species,
and immune and thyroid endpoints
The Workgroup discussed the uncertainty factors and
selected an uncertainty factor of 10 for database
deficiencies. Several items noted were that the available
studies were largely in one species, with no mouse or
non-human primate data, and that there was insufficient
information addressing immune or thyroid endpoints.
Toxicity
value
83,000 ng/kg/day (8.3 mg/kg/day)
Human equivalent dose divided by the total uncertainty
factor = toxicity value
15
Exposure
parameters
for drinking
water HBVs
95th percentile of water intake for consumers only (direct and indirect
consumption) for adults (>21 years old) of 3.353 L/day, per Table 3-1,
USEPA Exposure Factors Handbook, 2019.
An adult body weight of 80 kilograms was used (Table 8-1, USEPA
2011b).
A default Relative Source Contribution of 20% was included.
The Workgroup discussed the use of an upper percentile
water intake. The 95
th
percentile for consumers only was
selected as it would protect those drinking larger amounts
of water.
As no human serum data were available to assess the
population’s exposure to PFHxA from sources other than
drinking water, a default Relative Source Contribution of
20% was selected consistent with USEPA (2000)
guidance.
The Workgroup evaluated the protectiveness of the renal
tubular degeneration and renal papillary necrosis in
relation to the reduced pup weights observed in Loveless
et al. (2009).
Available data did not support Benchmark Dose Modeling
for further evaluation of Loveless et al. (2009) data.
Drinking
water HBV
400,000 ng/L (ppt) (400 micrograms per Liter or parts per billion)
Numeric HBV derived and justified using the above
information in the following equation:
=
× × ℎ
16
Chemical Summary for PFOS
Decision point
Rationale/justification
Critical
study
Dong GH, Zhang YH, Zheng L, Liu W, Jin YH, He QC. (2009). Chronic
effects of perfluorooctanesulfonate exposure on immunotoxicity in adult
male C57BL/6 mice. Arch Toxicol. 83(9):805-815.
The Workgroup discussed the available evaluations,
particularly MDH (2019) and New Jersey Department of
Environmental Protection (NJDEP) (2018), and selected a
critical study with an immune system functional assay
rather than observational data.
Description
of the critical
study
Adult male C57BL/6 mice were exposed to PFOS daily via oral gavage
for 60 days with 0, 0.5, 5, 25, 50 or 125 mg/kg total administered dose,
equivalent to 0 or approximately 0.008, 0.08, 0.4, 0.8 or 2.1 mg/kg/day.
The NOAEL for suppression of plaque forming cell response and
increase in liver mass was 0.5 mg/kg total administered dose which
corresponded to a serum concentration of 0.674 mg/L.
The Workgroup acknowledged that immune effects in
mice were seen at lower doses in Peden-Adams et al.
(2008). Serum concentrations from Peden-Adams et al.
(2008) were well below both the NOAEL and LOAEL
serum concentrations measured from several other
studies as described by Pachkowski et al. (2019) and may
be an outlier in the database.
Point of
Departure
The NOAEL for suppression of plaque forming cell response and
increase in liver mass was 0.5 mg/kg total administered dose which
corresponded to a serum concentration of 0.674 mg/L.
The Workgroup decided that serum-based points of
departure were appropriate for PFAS.
Human
equivalent
dose
The serum concentration of 0.674 mg/L was converted to the HED
using the below equation (based on ATSDR 2018).
NOAEL
HED
= (TWA serum x k
e
x V
d
) = 0.0000866 mg/kg/day
Ke = 0.000558539 (5.5 x 10
-4
) based on a human serum half-life of
1241 days (Li et al. 2018)
Vd = 0.23 L/kg (Thompson et al. 2010)
The Workgroup selected the serum half-life from a non-
occupationally exposed population as it is closer to the
general population’s exposure. The Workgroup selected
volume of distributions based on human data,
when available.
Uncertainty
factors
A total uncertainty factor of 30:
• 1 for LOAEL to NOAEL
• 10 for human variability
• 3 (10
0.5
) for animal to human difference (toxicodynamics)
• 1 for subchronic to chronic
• 1 for database deficiencies
The Workgroup reviewed the uncertainty factors selected
by MDH (2019) and adjusted the database uncertainty
factor to 1 based on the critical study selection. With
consideration of the selected immunotoxicity endpoint, the
database uncertainty factor of 1 was supported by the
assessments by USEPA (2016), NJDEP (2018), ATSDR
(2018) and New Hampshire (2019).
17
Toxicity
value
2.89 ng/kg/day (2.89 x 10
-6
mg/kg/day) which corresponds to a serum
concentration of 0.022 µg/ml
Serum levels used in development of these toxicity levels are not
meant to indicate a level where health effects are likely. These serum
levels are calculated to be at a point where no or minimal risk exists
for people drinking water with a certain PFAS.
Human equivalent dose or serum level divided by the total
uncertainty and modifying factors = toxicity value
Exposure
parameters
for drinking
water HBV
Breast-fed infant, which is also protective of a formula-fed infant
Placental transfer of 43% (MDHHS 2019)
Breastmilk transfer of 1.3% (MDHHS 2019)
Human serum half-life of 1241 days (3.2 years) (Li et al. 2018)
Volume of distribution of 0.23 L/kg (Thompson et al. 2010)
95th percentile drinking water intake, consumers only, from birth to
more than 21 years old (Goeden et al. [2019])
Upper percentile (mean plus two standard deviations) breast milk
intake rate (Goeden et al. [2019])
Time-weighted average water ingestion rate from birth to 30-35 years
of age (to calculate maternal serum concentration at delivery)
(Goeden et al. [2019])
Relative Source Contribution of 50%
Based on NHANES 95th percentiles for 3-11 (2013-2014) and over 12
years old (2015-2016) participants (CDC 2019)
The Workgroup discussed the Goeden et al. (2019) model
which considered full life stage exposure, from fetal
exposure, to infant exposure through breastfeeding, and
into adulthood. While the model was also developed for a
formula-fed infant, the breastfed infant scenario is
protective of a formula-fed infant. The Workgroup selected
this model for developing drinking water HBVs when the
needed inputs were available.
Drinking
water HBV
16 ng/L (ppt)
Numeric HBV derived and justified using the above
information
18
Chemical Summary for PFHxS
Decision point
Rationale/justification
Critical
study
NTP 2018 TOX-96: Toxicity Report Tables and Curves for Short-term
Studies: Perfluorinated Compounds: Sulfonates and personal
communication between MDH and NTP project manager Dr.
Chad Blystone (as cited in the HRA Toxicology Review Worksheet
for PFHxS, last revised 3/8/2019)
The Workgroup reviewed available evaluations and focused
on the ones from Minnesota Department of
Health (2019) and ATSDR (2018). In both evaluations,
thyroid endpoints were selected.
The Workgroup discussed Chang et al. (2018) and
concluded that the health outcome (reduction in litter size)
was a marginal effect.
Description
of the critical
study
28-day oral toxicity study in Sprague Dawley rats (NTP,
2018). PFHxS was administered via daily gavage at the following
doses for 28 continuous days:
Male rats: 0, 0.625, 1.25, 2.5, 5 or 10 mg/kg/day
Male rats mean measured plasma levels: 0.102, 66.76, 92.08, 129.0,
161.7, and 198.3 µg/ml
Female rats: 0, 3.12, 6.25, 12.5, 25, 50 mg/kg/day
Female rats mean measured plasma levels: 0.1754, 37.03, 50.41, 63.82,
83.82, and 95.51 µg/ml
n=10/sex/dose
Critical effect: decreased serum free thyroxin (T
4
) levels was
observed in adult male rats at the lowest PFHxS dose administered
(0.625 mg/kg/day)
Co-critical effects: decreased free and total T
4
, triiodothyronine (T
3
),
and changes in cholesterol levels and increased hepatic focal
necrosis
The Workgroup selected this thyroid endpoint as it was a
measure of a clinical or functional effect rather
than observational.
Point of
Departure
POD of 32.4 mg/L serum concentration for male rats based on
BMDL
20
. A BMR of 20% was used in the BMD modeling based on clinical
and toxicological knowledge regarding adverse outcomes associated with
decreases in circulating thyroid hormones. MDH stated that 20% provided
a more statistically reliable and biologically significant BMR. (MDH
conducted Benchmark Dose modeling and provided modeling run data in
the HRA Toxicology Review Worksheet for PFHxS, last revised
3/8/2019.
The Workgroup decided that serum-based points of
departure were appropriate for PFAS.
Although the Workgroup concluded that the Chang et al.
(2018) health outcome was marginal, they did note that the
serum concentration at the NOAEL for Chang et al. (2018)
was equivalent to the serum concentration at the selected
POD.
Human
equivalent
dose
The POD (32.4 mg/L) was multiplied by a toxicokinetic adjustment
based on the chemical’s specific clearance rate of 0.000090 L/kg-d
(Vd = 0.25 L/kg [Sundstrom et al. [2012], half-life = 1935 days [Li et al.
2018]) for a human equivalent dose of 0.00292 mg/kg/day.
The Workgroup selected the human serum half-life from Li
et al. (2018) as it was a non-occupational population
drinking water with elevated PFAS.
19
Uncertainty
factors
Total Uncertainty Factor of 300
• 1 for LOAEL to NOAEL
• 10 for human variability
• 3 (10
0.5
) for animal to human variability (toxicodynamic
differences)
• 1 for subchronic to chronic
• 10 for database deficiencies - to address concerns for early life
sensitivity and lack of 2-generation or immunotoxicity studies
The Workgroup reviewed the uncertainty factors used by
MDH (2019) and concluded that the database uncertainty
factor of 10 was very defensible in this situation, especially
for the lack of information on early-life sensitivity.
Toxicity
value
9.7 ng/kg/day (9.7 x 10
-6
mg/kg/day) which corresponds to a serum
concentration of 0.11 µg/ml
Serum levels used in development of these toxicity levels are not
meant to indicate a level where health effects are likely. These serum
levels are calculated to be at a point where no or minimal risk exists
for people drinking water with a certain PFAS.
Human equivalent dose or serum level divided by the total
uncertainty factors = toxicity value
Exposure
parameters
for drinking
water HBV
Breast-fed infant, which is also protective of a formula-fed infant
Placental transfer of 80% (MDHHS 2019)
Breastmilk transfer of 1.2% (MDHHS 2019)
Human serum half-life of 1935 days (Li et al. [2018])
Volume of distribution of 0.25 L/kg (MDH [2019] based on
Sundstrom et al. [2012])
95
th
percentile drinking water intake, consumers only, from birth to more
than 21 years old (Goeden et al. [2019])
Upper percentile (mean plus two standard deviations) breast milk
intake rate (Goeden et al. [2019])
Time-weighted average water ingestion rate from birth to 30-35 years
of age (to calculate maternal serum concentration at
delivery) (Goeden et al. [2019])
Relative Source Contribution of 50% (0.5)
Based on NHANES 95
th
percentiles for 3-11 (2013-2014) and over 12
years old (2015-2016) participants (CDC 2019)
The Workgroup discussed the Goeden et al.
(2019) model which considered full life stage exposure, from
fetal exposure, to infant exposure through breastfeeding,
and into adulthood. While the model was also developed for
a formula-fed infant, the breastfed infant scenario is
protective of a formula-fed infant. The Workgroup selected
this model for developing drinking water HBVs when
the needed inputs were available.
Drinking
water HBV
51 ng/L (ppt)
Numeric HBV derived and justified using the above
information
20
Chemical Summary for PFBS
Decision point
Rationale/justification
Critical
study
Feng, X; Cao, X; Zhao, S; Wang, X; Hua, X; Chen, L; Chen, L. (2017).
Exposure of pregnant mice to perfluorobutanesulfonate causes
hypothyroxinemia and developmental abnormalities in female
offspring. Toxicol Sci 155: 409-419.
The Workgroup evaluated available agency decision
documents and selected the study associated with the draft
USEPA (2018) PFBS toxicity value based on thyroid effects.
The kidney effects identified in the draft USEPA (2018)
toxicity assessment were identified as a potentially
compensatory response. The thyroid effects were
identified as having greater functional significance.
Description
of the critical
study
PFBS was orally administered to pregnant ICR mice (n=30/dose) at
doses of 0, 50, 200, and 500 mg/kg/day from gestational day (GD) 1 to
GD20. Dams (F0) and female offspring (F1) from each dose
group were subsequently evaluated for 1) growth and development, 2)
hormone levels, and 3) serum PFBS levels. The critical effect is
decreased serum total thyroxine (T
4
) in newborn (PND 1) mice.
Selection of total T
4
as the critical effect is based on a several key
considerations that account for cross-species correlations in thyroid
physiology and hormone dynamics particularly within the context of a
developmental life stage.
Point of
Departure
A POD of 28.19 mg/kg/day (BMDL
20
) for decreased serum total T
4
in
newborn (PND 1) mice was selected
The Workgroup noted that a Benchmark Dose approach is
preferable to a NOAEL/LOAEL.
The Workgroup noted that the thyroid point of departure
would be protective of the kidney effects as well.
The draft USEPA (2018) toxicity assessment contained
administered doses from the individual studies converted to
HED doses using study-specific Dosimetric Adjustment
Factors (DAF; not reported for each dosing group) derived
using allometric scaling (BW
3/4
) prior to BMD model
analysis.
An example DAF calculation was provided in Table 8 of the
draft USEPA (2018) toxicity assessment: dose x DAF = 200
x 0.149 = 29.9 mg/kg/day, where DAF equals
(BW
animal
1/4
)/(BW
human
1/4
) = 0.0399
1/4
÷ 80
1/4
= 0.149
The POD
HED
= 4.2 mg/kg/day for decreased serum total T
4
in
newborn (PND 1) mice (USEPA 2018).
The USEPA POD
HED
of 4.2 was divided by 0.149 (USEPA
example DAF) to obtain a BMDL
20
of 28.19 mg/kg/day.
21
Human
equivalent
dose
The BMDL
20
-HED is 0.0892 mg/kg/day.
The BMDL
20
of 28.19 mg/kg/day was divided by the Dose Adjustment
Factor of 316 (human serum half-life/female mouse serum half-life =
665 hours/2.1 hours = 316) (MDH, 2017).
The Workgroup evaluated the half-life based Dose
Adjustment Factor used by the Minnesota Department of
Health (MDH) (2017). As that allowed conversion of the
point of departure to a human equivalent dose using
chemical-specific information, the Workgroup selected this
approach over the allometric scaling used in the draft
USEPA (2018) PFBS toxicity assessment.
Uncertainty
factors
The total uncertainty factor is 300.
• 1 for LOAEL to NOAEL
• 10 for human variability
• 3 (10
0.5
) for animal to human variability
• 1 for subchronic to chronic
• 10 for database deficiencies, for the lack of
neurodevelopmental, immunotoxicological, and chronic studies
The Workgroup discussed the uncertainty factors selected
in the draft USEPA (2018) toxicity assessment and
supported their use.
Toxicity
value
300 ng/kg/day (0.0003 mg/kg/day)
Human equivalent dose or serum level divided by the total
uncertainty factors = toxicity value
Exposure
parameters
for drinking
water HBV
95
th
percentile of water intake for consumers only (direct and indirect
consumption) for infants (birth to <1 year old) of 1.106 L/day, per
Table 3-1, USEPA Exposure Factors Handbook, 2019.
An infant body weight of 7.8 kilograms was used and represents a
time-weighted average for birth to 1 year old (Table 8-1, USEPA
2011).
A default Relative Source Contribution of 20% was included.
The Workgroup discussed the use of an upper percentile
water intake. The 95
th
percentile for consumers only was
selected as it would protect those drinking larger amounts of
water.
As insufficient human serum data was available to assess
the population’s exposure to PFBS from sources other than
drinking water, a default Relative Source Contribution of
20% was selected consistent with USEPA (2000) guidance.
Drinking
water HBV
420 ng/L (ppt)
Numeric HBV derived and justified using the above
information in the following equation:
=
× × ℎ
22
Chemical Summary for GenX
Decision point
Rationale/justification
Critical
study
Oral (Gavage) Reproduction/ Developmental Toxicity Study in Mice
(OECD TG 421; modified according to the Consent Order) DuPont-
18405-1037 (2010) (also contains 90-day toxicity study information
and outcomes - that information is not described here)
The Workgroup evaluated the North Carolina Department
of Health and Human Services (2017) and draft USEPA
(2018) information. The draft USEPA (2018) evaluation
was identified as providing a more in-depth and robust
analysis and approach.
Description
of the critical
study
In a combined oral gavage reproductive/developmental toxicity study
in mice with HFPO dimer acid ammonium salt, the test compound was
administered by oral gavage to Crl:CD1(ICR) mice (25/sex/group) at
doses of 0, 0.1, 0.5, or 5 mg/kg/day, according to a modified OECD
TG 421. Parental F0 males were dosed 70 days prior to mating and
throughout mating through 1 day prior to scheduled termination.
Parental F0 females were dosed for 2 weeks prior to pairing and were
dosed through LD 20. F1 animals (offspring) were dosed daily
beginning on PND 21 through PND 40.
At 0.5 mg/kg/day, liver effects (increased absolute and relative weight
and histopathologic findings) were reported in both males and
females.
At 5 mg/kg/day, male and female F1 pups exhibited lower mean BWs
at PNDs 4, 7, 14, 21, and 28. Male F1 pups continued to exhibit lower
mean BWs at PNDs 35 and 40. The USEPA (2018) identified
additional developmental effects (delays in balanopreputial separation
and vaginal patency) that occurred at the same dose level, but the
biological significance of these effects are equivocal as described.
NOAEL (F0) = 0.1; LOAEL (F0) = 0.5 for liver effects (single-cell
necrosis in males, and increased relative liver weight in both sexes).
NOAEL (F1) = 0.5 for developmental effects (decreased pup
weights).
The Workgroup noted that while primarily industry-funded
studies are the only ones available, they followed
recognized testing guidelines and/or were published
following external peer-review. These studies appear to be
sufficient for developing values.
23
Point of
Departure
BMDL
10
= 0.15 mg/kg/day for liver single cell necrosis in parental
males (DuPont-18405-1037, 2010).
The Workgroup noted that the Benchmark Dose approach
is preferred over the use of a NOAEL/LOAEL.
USEPA (2018) evaluated the relevance of this endpoint in
humans and noted that, per the Hall criteria (Hall et al.,
2012) liver effects accompanied by effects such as
necrosis or inflammation, among others, are indicative of
liver tissue damage (USEPA, 2018).
While some liver effects in rodents are mediated through
PPARα and may be less relevant to humans, available
information indicates that liver single cell necrosis may be
mediated by a number of processes and pathways.
In PPARα-mediated rodent hepatocarcinogenesis, liver
necrosis is not a key event. (DeWitt and Belcher, 2018)
Human
equivalent
dose
A candidate POD
HED
was derived from the BMDL
10
for liver
effects using a BW
3/4
allometric scaling approach. A BW
a
of
0.0372
kg was identified as the mean BW of the F0 male mouse controls.
A BW
h
of 80 kg for humans was selected. The resulting DAF for
the allometric scaling of doses from mice to humans is 0.15. Using
the BMDL
10
of 0.15 mg/kg/day to complete the calculation results
in a POD
HED
for single-cell necrosis of the liver from DuPont-
18405-1037 (2010) of 0.023 mg/kg/day (USEPA 2018).
The Workgroup noted that a toxicokinetic adjustment from
the point of departure to human equivalent dose would
provide a chemical-specific conversion. However, no
chemical-specific data on human serum half-life was
available that would allow this conversion. Allometric
scaling, per USEPA (2011a) guidance, was used.
Uncertainty
factors
Total Uncertainty Factor of 300
• 1 for use of a LOAEL to NOAEL
• 10 for human variability
• 3 (10
0.5
) for animal to human variability
• 3 (10
0.5
) for subchronic-to-chronic
• 3 (10
0.5
) for database deficiencies, including lack of
epidemiological, and developmental
and immunotoxicological studies in laboratory animals
The Workgroup evaluated the uncertainty factors selected
by USEPA (2018). Given the deficiencies in the database,
including a lack of epidemiological studies and
developmental and immunotoxicological in laboratory
animals, a database uncertainty factor of 3 was retained.
In conjunction with the deficiencies covered by the
database uncertainty factor, the subchronic to chronic
uncertainty factor of 3 was identified as sufficient.
Toxicity
value
77 ng/kg/day (7.7 x10-5 mg/kg/day)
Human equivalent dose or serum level divided by the total
uncertainty = toxicity value
24
Exposure
parameters
for drinking
water HBV
95
th
percentile of water intake for consumers only (direct and
indirect consumption) for adults (>21 years old) of 3.353 L/day
, per
Table 3-1, USEPA Exposure Factors Handbook, 2019.
An adult body weight of 80 kilograms was used (Table 8-1,
USEPA 2011b).
A default Relative Source Contribution (RSC) of 20% was
included.
The Workgroup discussed the use of an upper percentile water
intake. The 95
th
percentile for consumers only was selected as it
would protect those drinking larger amounts of water.
As no human serum data was available to assess the population’s
exposure to GenX from sources other than drinking water, a
default Relative Source Contribution of 20% was
selected consistent with USEPA (2000) guidance.
The Workgroup evaluated the protectiveness of adult exposure in
combination with the point of departure. The NOAEL for
developmental effects described above was at a dose five times
higher than the NOAEL for liver necrosis effects. As a drinking
water value based on the developmental NOAEL would be higher
than the level presented below, the Workgroup decided that the
drinking water HBV below based on liver effects would be
sufficiently conservative to be protective of infant exposure.
Drinking
water HBV
370 ng/L (ppt)
Numeric HBV derived and justified using the above information in
the following equation:
=
× × ℎ
25
Rationale for Individual HBVs
While there are on-going discussions regarding the grouping of multiple PFAS into one drinking
water value, there is no consensus from the scientific community on which PFAS should be
grouped or the basis of that grouping. Grouping methods that have been applied include
combining multiple PFAS into one number based on known or assumed toxicity, carbon chain
length, and/or biological half-life (simple addition) as well as the use of relative ability of the
grouped PFAS to lead to a comparable health endpoint (toxic equivalency); the latter approach
being similar to those used for dioxins, furans, and coplanar polychlorinated biphenyls.
There is, however, scientific agreement that the long-chain PFAS (eight carbons and above for
carboxylates and six carbons and above for sulfonates) have similar toxicity. Based on the
similarity in toxicity for the long-chain PFAS, the Workgroup recommends use of the HBV for
PFNA (6 ng/L [ppt]) as a screening level for all other long-chain PFAS included on the USEPA
Method 537.1 analyte list for which the Workgroup did not develop an individual HBV. This
screening level should not be used to evaluate the risk of developing health effects, but as a
screening tool for EGLE/public water supplies to use for decision making.
Adverse health effects of long chain (six-carbon perfluorosulfonic acids or eight-carbon
perfluorocarboxylic acids) have been established in epidemiological and laboratory animal model
studies. These adverse health effects include kidney and testicular cancer, elevated serum
cholesterol, endocrine effects, immune effects, and reproductive effects (ATSDR, 2018). These
effects are supported by studies of different human populations exposed to a few or to many
PFAS, including those from populations of high PFAS exposure and the general population and
demonstrate that many different long-chain PFAS can produce similar adverse health effects in
exposed humans. However, while not all long-chain PFAS have robust data available for the
development of a HBV, the totality of evidence indicates that long-chain PFAS in drinking water
may pose risks of adverse health effects.
While health concerns are based on the total exposure to PFAS across many sources, because
drinking water is the predominant source of exposure for many people consuming contaminated
water, it remains the focus for health-based regulation based on current knowledge. Therefore,
monitoring of drinking water should continue and be based on levels that will be protective for
exposure to all PFAS.
At this time, it is recommended that the proposed HBV for PFNA be used as a screening level for
the long chain PFAS included in USEPA Method 537.1 that may be found in drinking water that
are not covered by an individual PFAS HBVs as presented in the Summary Table of Drinking
Water HBVs.
26
Summary of Conclusions
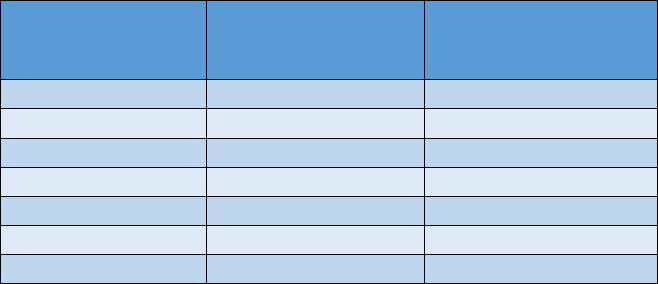
Summary Table of Drinking Water HBVs
Specific PFAS
Drinking Water
Health-based
Value
Chemical Abstract
Services Registry
Number (CASRN)
PFNA
6 ng/L (ppt)
375-95-1
PFOA
8 ng/L (ppt)
335-67-1
PFHxA
400,000 ng/L (ppt)
307-24-4
PFOS
16 ng/L (ppt)
1763-23-1
PFHxS
51 ng/L (ppt)
355-46-4
PFBS
420 ng/L (ppt)
375-73-5
GenX
370 ng/L (ppt)
13252-13-6
For all other PFAS on the USEPA Method 537.1 analyte list, the Workgroup recommendation is
to use the lowest long-chain (eight carbons and above for carboxylates and six carbons and above
for sulfonates) HBV of 6 ppt, which is the HBV for PFNA. Those other long-chain PFAS included
in USEPA Method 537.1 are: NEtFOSAA (CASRN: 2991-50-6); NMeFOSAA (CASRN: 2355-31-
9); PFDA (CASRN: 335-76-2); PFDoA (CASRN: 307-55-1); PFTA (CASRN: 376-06-7); PFTrDA
(CASRN: 72629-94-8); and PFUnA (CASRN: 2058-94-8).
As shown in Figure 1 (below), the drinking water values for PFOS and PFOA have gone down
over time. This is a reflection of the evolving science, both the ever-increasing knowledge gained
from published toxicology and epidemiology studies and the risk assessments for development
of toxicity values and drinking water values. Information continues to become available on multiple
PFAS and as there are thousands of PFAS, new information will likely become available for many
years to come. It is quite possible that the same trend demonstrated in Figure 1 will be seen for
other PFAS, where drinking water values become lower over time and that new values could be
developed within a few years’ time. As described in the Challenges and Limitations section, along
with use of current scientific data, development of drinking water values includes a certain amount
of scientific judgement informed from the scientific knowledgebase. It is that combination of
scientific judgement and data that ultimately informs the development of drinking water values.
With emerging contaminants like PFAS, rapid availability of data drives public health protective
actions and drinking water values.
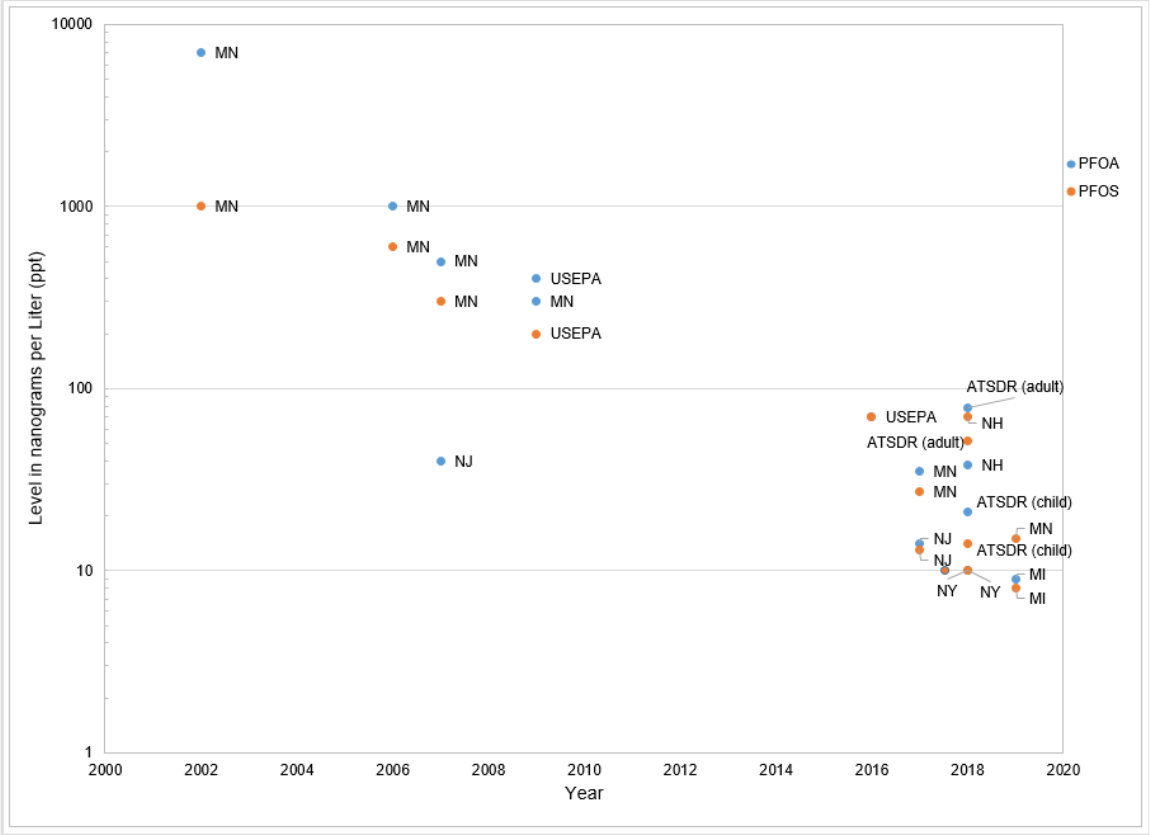
27
PFOS and PFOA
Figure 1: Screening Levels, Health-Based Values, and Regulatory Standards for PFOS and PFOA Over a 20-Year Timeframe.
The numbers in Figure 1 are the various screening levels, HBVs, and regulatory standards
developed by various agencies and states over time as of June 2019. It does not include the
agencies that include multiple PFAS into a single value. This should not be considered an
exhaustive list of all PFAS drinking water values available, and values may be updated, and
additional values will likely become available. The Michigan values included in Figure 1 are the
MPART Human Health Workgroup public health drinking water screening levels.
Concluding Remarks
The Workgroup would like to commend the State of Michigan for addressing PFAS concerns with
unusual rigor, openness, and reliance on independent scientific guidance. From the beginning of
the recognition of environmental and public health issues related to PFAS, the State of Michigan
has been at the forefront nationally in assessing the scope of the contamination, intervening to
mitigate exposure, and monitoring the evidence to guide policy. The statewide survey of drinking
28
water supplies was highly unusual if not unique relative to other areas, and the process of
developing Maximum Contaminant Levels as rigorous as any in the nation. By engaging experts
from outside the state agencies to complement the considerable expertise of the staff in the
Michigan Departments of Health and Human Services and Environment, Great Lakes, and
Energy, they have demonstrated their commitment to following the evidence through to
developing sound policy.
29
References
ATSDR. (2018). Agency for Toxic Substances and Disease Registry. Toxicological Profile for
Perfluoroalkyls. Draft for Public Comment. June 2018.
Bartell SM, Calafat AM, Lyu C, et al. 2010. Rate of decline in serum PFOA concentrations after
granular activated carbon filtration at two public water systems in Ohio and West Virginia.
Environ Health Perspect 118(2):222-228.
Beck, N.B., Becker, R.A., Erraguntla, N., Farland, W.H., Grant, Gray, G., Kirman, C., LaKind,
J.S., Lewis, R.J., Nance, P., Pottenger, L.H., Santos, S.L., Shirley, S., Simon, T., and Dourson,
M.L. (2016). Approaches for describing and communicating overall uncertainty in toxicity
characterizations: U.S. Environmental Protection Agency’s Integrated Risk Information System
(IRIS) as a case study. Environment International 89-90, 110-128.
Blystone, C. (2019). [Personal Communication. Use of NTP data tables and study protocol
(January 2019 email exchange).]., cited in Minnesota Department of Health (2019).
Toxicological Summary for Perfluorohexane sulfonate
. Accessed online June 2019 at:
CDC. (2019). (Center for Disease Control)
Fourth National Report on Human Exposure to
Environmental Chemicals, Updated Tables, January 2019, Volume One.
Chang, S., JL Butenhoff, GA Parker, PS Coder, JD Zitsow, RM Krisko, JA Bjork, KB Wallace,
JG Seed. (2018). Reproductive and developmental toxicity of potassium
perfluorohexanesulfonate in CD-1 mice. Reproductive Toxicology, 78, 150-168.
Das KP, Grey BE, Rosen MB, et al. 2015. Developmental toxicity of perfluorononanoic acid in
mice. Reproductive Toxicology 51:133-144.
DeWitt and Belcher (2018)
Memorandum from Jamie DeWitt and Scott Belcher to the members
of the North Carolina Secretaries’ Science Advisory Board. Accessed online June 2019.
Dong GH, Zhang YH, Zheng L, Liu W, Jin YH, He QC. (2009). Chronic effects
of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice.
Arch Toxicol. 83(9):805-815.
DuPont-18405-1037: E.I. du Pont de Nemours and Company (2010) An Oral (Gavage)
Reproduction/Developmental Toxicity Screening Study of H-28548 in Mice. USEPA OPPTS
870.3550; OECD Test Guideline 421. Study conducted by WIL Research Laboratories, LLC
(Study Completion Date: December 29, 2010), Ashland, OH. (as cited in USEPA (2018)).
Feng, X; Cao, X; Zhao, S; Wang, X; Hua, X; Chen, L; Chen, L. (2017). Exposure of pregnant
mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in
female offspring. Toxicol Sci 155: 409-419.
Goeden HM, Greene CW, Jacobus, JA (2019) A transgenerational toxicokinetic model and its
use in derivation of Minnesota PFOA water guidance. J. Exposure Sci. Env. Epidemiol. 29:183-
195.

30
Goeden, HM., CW Greene, JA Jacobus. (2019). A transgenerational toxicokinetic model and its
use in derivation of Minnesota PFOA water guidance. Journal of Exposure Science &
Environmental Epidemiology.
Hall, AP, Elcombe, CR, Foster, JR, et al. (2012) Liver Hypertrophy: A Review of Adaptive
(Adverse and Non-adverse) Changes—Conclusions from the 3rd International ESTP Expert
Workshop. Toxicologic Pathol. 40: 971-94.
Klaunig, JE, Shinohara, M, Iwai, H, Chengelis, CP, Kirkpatrick, JB, Wang, Z, Bruner, RH (2015)
Evaluation of the chronic toxicity and carcinogenicity of perfluorohexanoic acid (PFHxA) in
Sprague-Dawley rats. Toxicol. Pathol. 43 (2), 209–220.
Koskela A, Finnilä MA, Korkalainen M, Spulber S, Koponen J, Håkansson H, Tuukkanen J,
Viluksela M. (2016) Effects of developmental exposure to perfluorooctanoic acid (PFOA) on
long bone morphology and bone cell differentiation. Toxicol Appl Pharmacol. 301:14-21.
Li, Y., T Fletcher, D Mucs, K Scott, CH Lindh, P Tallving, K Jakobsson. (2018). Half-lives of
PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational
and Environmental Medicine, 75, 46-51.
Loveless, S.E., Slezak, B., Serex, T., Lewis, J., Mukerji, P., O'Connor, J.C., Donner, E.M.,
Frame, S.R., Korzeniowski, S.H., Buck, R.C., 2009. Toxicological evaluation of
sodium perfluorohexanoate. Toxicology 264 (1–2), 32–44.
Luz, AL, Anderson, JK, Goodrum, P, Durda, J. (2019) Perfluorohexanoic acid toxicity, part I:
Development of chronic human health toxicity value for use in risk assessment.
Reg. Toxicol. Pharmacol. 103: 41-55.
Minnesota Department of Health (2019).
Toxicological Summary for Perfluorooctane sulfonate
(PFOS). Accessed online June 2019.
Minnesota Department of Health (2017).
Toxicological Summary for: Perfluorobutane Sulfonate
(PFBS). Accessed online June 2019.
Minnesota Department of Health (2017). Background Document: Toxicokinetic Model for PFOS
and PFOA and Its Use in the Derivation of Human Health-based Water Guidance Values.
Minnesota Department of Health (2019). HRA Toxicology Review Worksheet for PFHxS, last
revised 3/8/2019.
North Carolina Department of Health and Human Services.
Questions and Answers Regarding
North Carolina Department of Health and Human Services Updated Risk Assessment for GenX
(Perfluoro-2-propoxypropanoic acid). July 14, 2017. Accessed online May 2019.
National Research Council (NRC). (2009). Science and Decisions: Advancing Risk Assessment.
Washington D.C.: National Academies Press.
NTP. (2018). National Toxicology Program. TOX-96: Toxicity Report Tables and Curves for
Short-term Studies: Perfluorinated Compounds: Sulfonates. Retrieved from
https://tools.niehs.nih.gov/cebs3/views/?action=main.dataReview&bin_id=3874., cited
31
in Minnesota Department of Health (2019). Toxicological Summary
for Perfluorohexane sulfonate. Accessed online June 2019 at:
https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/pfhxs.pdf.
New Jersey DEP DWQI. (2018).
Appendix A - Health-based Maximum Contaminant Level
Support Document: Perfluorooctane sulfonate. Trenton, NJ: New Jersey Drinking Water Quality
Institute.
New Jersey DEP DWQI. (2015).
Maximum contaminant level recommendation
for perfluorononanoic acid in drinking water. Trenton, NJ: New Jersey Drinking Water Quality
Institute.
Ohmori K, Kudo N, Katayama K, et al. 2003. Comparison of
the toxicokinetics between perfluorocarboxylic acids with different carbon chain length.
Toxicology 184:135-140.
Onishchenko N, Fischer C, Wan Ibrahim WN, Negri S, Spulber S, Cottica D, Ceccatelli S.
(2011) Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related
manner. Neurotox Res. 19(3):452-61.
Pachkowski B, Post GB, Stern AH. (2019)
The derivation of a Reference Dose (RfD) for
perfluorooctane sulfonate (PFOS) based on immune suppression. Environ Res. 2019 171:452-
469.
Peden-Adams, MM, Keller, JM, EuDaly, JM, Berger, J, Gilkeson, GS, Keil, DE.
(2008) Suppression of Humoral Immunity in Mice following Exposure
to Perfluorooctane Sulfonate. Toxicol. Sci. 104(1): 144-154.
Russell, MH, Nilsson, H, Buck, RC (2013) Elimination kinetics of perfluorohexanoic acid in
humans and comparison with mouse, rat and monkey. Chemosphere. 93: 2419-2425.
Sundstrom, M., SC Chang, PE Noker, GS Gorman, JA Hart, DJ Ehresman, A Bergman,
JL Butenhoff. (2012). Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in
rats, mice, and monkeys. Reproductive Toxicology, 33, 441-451.
Thompson, J., M. Lorber, L.-M.L. Toms, K. Kato, A.M. Calafat, and J.F. Mueller. 2010. Use of
simple pharmacokinetic modeling to characterize exposure of Australians to perfluorooctanoic
acid and perfluorooctane sulfonic acid. Environment International 36:390–397.
United States Environmental Protection Agency (2000).
Methodology for Deriving Ambient
Water Quality Criteria for the Protection of Human Health. EPA-822-B-00-004. USEPA Office of
Water, Washington, DC. Accessed online June 2019.
United States Environmental Protection Agency. (2002).
A Review of the Reference Dose and
Reference Concentration Processes. Risk Assessment Forum. EPA/630/P-02/002F, December
2002.
United States Environmental Protection Agency. (2011)
Exposure Factor Handbook: 2011
Edition. EPA/600/R-09/052F. USEPA, National Center for Environmental Assessment, Office of
Research and Development, Washington, DC. Accessed online June 2019.
32
United States Environmental Protection Agency (2011a). Recommended Use of Body Weight
3/4
as the Default Method in Derivation of the Oral Reference Dose. EPA/100/R11/0001. USEPA,
Office of the Science Advisor, Risk Assessment Forum, Washington, DC. Accessed online May
2018.
United States Environmental Protection Agency (2011b).
Exposure Factors Handbook 2011
Edition. EPA/600/R-09/052F. USEPA, National Center for Environmental Assessment, Office of
Research and Development, Washington, DC. Accessed online May 2019.
United States Environmental Protection Agency (2018).
Human Health Toxicity Values
for Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound
Potassium Perfluorobutane Sulfonate (CASRN 29420-49-3). Public Comment Draft. EPA-823-
R-18-307. USEPA, Office of Research and Development, Washington, DC. Accessed online
June 1, 2019.
United States Environmental Protection Agency (2018).
Human Health Toxicity Values for
Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6
and CASRN 62037-80-3), Also Known as “GenX Chemicals”. Public Comment Draft. EPA-823-
P-18-001. USEPA Office of Water, Washington, DC. Accessed online May 2019.
United States Environmental Protection Agency (2019)
Exposure Factors Handbook Chapter 3
(Update): Ingestion of Water and Other Select Liquids. EPA/600/R-18/259F. USEPA Office of
Research and Development, Washington, DC. Accessed online June 2019.
Wambaugh JF, Setzer RW, Pitruzzello AM, et al. 2013. Dosimetric anchoring of in vivo and in
vitro studies for perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci 136(2):308-327.
Zhang Y, Beesoon S, Zhu L, et al. 2013. Biomonitoring of perfluoroalkyl acids in human urine
and estimates of biological half-life. Environ Sci Technol 47(18):10619-10627.
33
Appendix A: Acronym List
ATSDR Agency for Toxic Substances and Disease Registry
BMD benchmark dose
BMDL lower confidence limit on the benchmark dose
BMR benchmark response
BW body weight
BWa body weight animal
BWh body weight human
CDC Centers for Disease Control and Prevention
DAF dosimetric adjustment factor
EGLE Environment, Great Lakes, and Energy (Michigan Department of)
GD gestational day
GenX perfluoro-2-propoxypropanoic acid
HBV health-based value
HED human equivalent dose
HFPO hexafluoropropylene oxide
HRA health risk assessment
kg kilogram
L liter
LD lactation day
LHA lifetime health advisory
LOAEL lowest observed adverse effect level
MCL Maximum Contaminant Level
MDH Minnesota Department of Health
MDHHS Michigan Department of Health and Human Services
mg milligram
MI Michigan
ml milliliter
MPART Michigan PFAS Action Response Team
µg microgram
ng nanogram
NHANES National Health and Nutrition Examination Survey
NJDEP New Jersey Department of Environmental Protection
NOAEL no observed adverse effect level
OECD Organization for Economic Co-operation and Development
PFAS per- and polyfluoroalkyl substances
PFBS perfluorobutane sulfonic acid
PFHxA perfluorohexanoic acid
PFHxS perfluorohexane sulfonic acid
PFNA perfluorononanoic acid
PFOA perfluorooctanoic acid
PFOS perfluorooctane sulfonic acid
PND postnatal day
POD point of departure
POD
HED
point of departure human equivalent dose
PPAR peroxisome proliferator-activated receptor
ppt parts per trillion
RfD reference dose
RSC relative source contribution
TWA time weighted average
UF uncertainty factor
USEPA United States Environmental Protection Agency
34
Appendix B: MPART Motion for Creation of Science Advisory Workgroup,
April 4, 2019
Motion
Motion to establish a Science Advisory Workgroup with the Charge described below, comprised
of external members with expertise in toxicology, epidemiology, and risk assessment, and
further to authorize the chairperson of MPART to finalize the appointments in consultation with
MPART members.
Preamble
On March 26, 2019, Governor Whitmer directed the Michigan PFAS Action Response Team
(MPART) to further protect public health and the environment, by forming a Science Advisory
Workgroup to “review both existing and proposed health-based drinking water standards from
around the nation to inform the rule making process for appropriate Maximum Contaminant
Levels for Michigan...” Toward this objective, the Science Advisory Workgroup shall make
numeric recommendation(s) to MPART for those per- and polyfluoroalkyls substances (PFAS)
for which adequate information exists.
Charge
The Science Advisory Workgroup shall:
1. For the PFAS listed in USEPA Method 537.1, review all existing and proposed national-
and state-derived PFAS drinking water standards and identify the most scientifically
defensible non-cancer or cancer-based public health toxicity values available for each
individual PFAS chemical family member, or combination thereof, for which the Science
Advisory Workgroup determines that adequate information exists. Provide written
justification that shall include, but not be limited to, the basis for the selection of the
primary study, critical effect identification, point of departure determination, evaluation of
all uncertainty and/or modification factors applied, and the non-cancer or cancer-based
toxicity value derivation.
2.
Review all existing and proposed national- and state-derived PFAS drinking water
standards and identify the most scientifically defensible exposure assessment and risk
evaluation methodology for each individual PFAS chemical family member, or
combination thereof, for which the Science Advisory Workgroup determines that
adequate information exists. Provide written justification that shall include, but not be
limited to, selection of the most appropriate receptor(s) and identification of all
appropriate exposure assumptions for the receptor(s).
3.
Identify the most appropriate and scientifically defensible combination of each specific
PFAS toxicity value and exposure assessment and risk evaluation methodology,
including consideration of relative source contribution, from which to derive a health-
based drinking water value for each individual PFAS chemical family member, or
combination thereof, for which the Science Advisory Workgroup determines that
adequate information exists.
4.
Provide to MPART no later than July 1, 2019, a report recommending scientifically-
defensible numeric health-based values to inform the rulemaking process for Maximum
Contaminant Levels for each individual PFAS chemical family member, or combination
thereof, with written justification for the calculation methodology and each input into used
in the methodology by the Science Advisory Workgroup.
End
35
Appendix C: USEPA Method 537.1 Analyte List
Analyte Name* Acronym
Fluorinated
Carbon Chain
Length
Chemical Abstract
Services Registry
Number (CASRN)
Perfluorotetradecanoic acid
PFTeA
C
14
376-06-7
Perfluorotridecanoic acid
PFTriA
C
13
72629-94-8
Perfluorododecanoic acid
PFDoA
C
12
307-55-1
Perfluoroundecanoic acid
PFUnA
C
11
2058-94-8
Perfluorodecanoic acid
PFDA
C
10
335-76-2
Perfluorononanoic acid
PFNA
C
9
375-95-1
Perfluorooctanoic acid
PFOA
C
8
335-67-1
Perfluoroheptanoic acid
PFHpA
C
7
375-85-9
Perfluorohexanoic acid
PFHxA
C
6
307-24-4
Perfluorooctanesulfonic acid
PFOS
C
8
1763-23-1
Perfluorohexanesulfonic acid
PFHxS
C
6
355-46-4
Perfluorobutanesulfonic acid
PFBS
C
4
375-73-5
2-(N-Ethylperfluorooctanesulfonamido)
acetic acid
N-EtFOSAA
C
8
2991-50-6
2-(N-Methylperfluorooctanesulfonamido)
acetic acid
N-MeFOSAA
C
8
2355-31-9
Hexafluoropropylene oxide dimer acid
HFPO-DA
(GenX)
C
6
13252-13-6
a
11-chloroeicosafluoro-3-oxaundecane-1-
sulfonic acid
11Cl-PF3OUdS
C
10
763051-92-9
b
9-chlorohexadecafluoro-3-oxanone-1-
sulfonic acid
9Cl-PF3ONS
C
8
756426-58-1
c
4,8-dioxa-3H-perfluorononanoic acid
ADONA
C
7
919005-14-4
d
a
HFPO-DA is one component of the GenX processing aid technology.
b
11Cl-PF3OUdS is available in salt form (e.g. CASRN of potassium salt is 83329-89-9).
c
9Cl-PF3ONS analyte is available in salt form (e.g. CASRN of potassium salt is 73606-19-6)
d
ADONA is available as the sodium salt (no CASRN) and the ammonium salt (CASRN is 958445-448).
* Some PFAS are commercially available as ammonium, sodium, and potassium salts. This method measures all
forms of the analytes as anions while the counterion is inconsequential. Analytes may be purchased as acids or as
any of the corresponding salts.
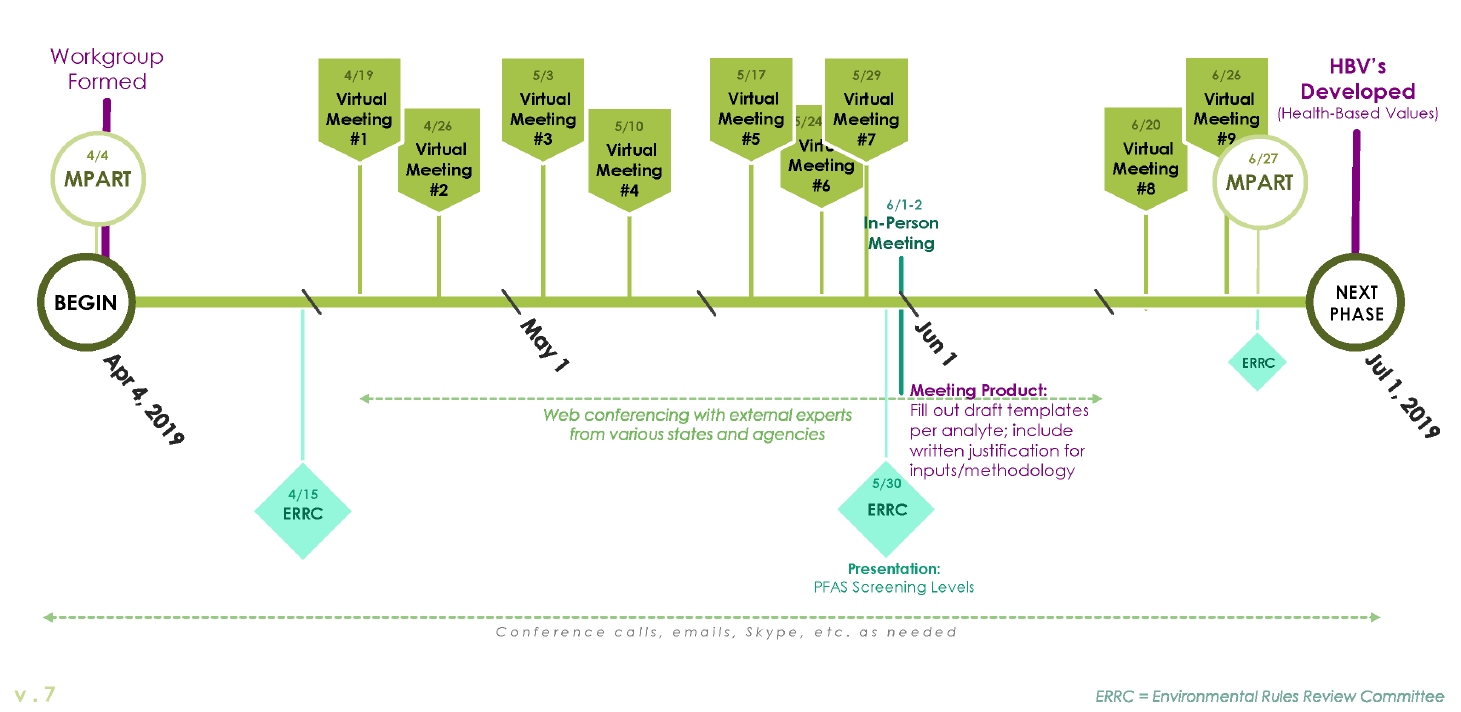
36
Appendix D: Timeline for the Science Advisory Workgroup’s Development of Drinking Water HBVs
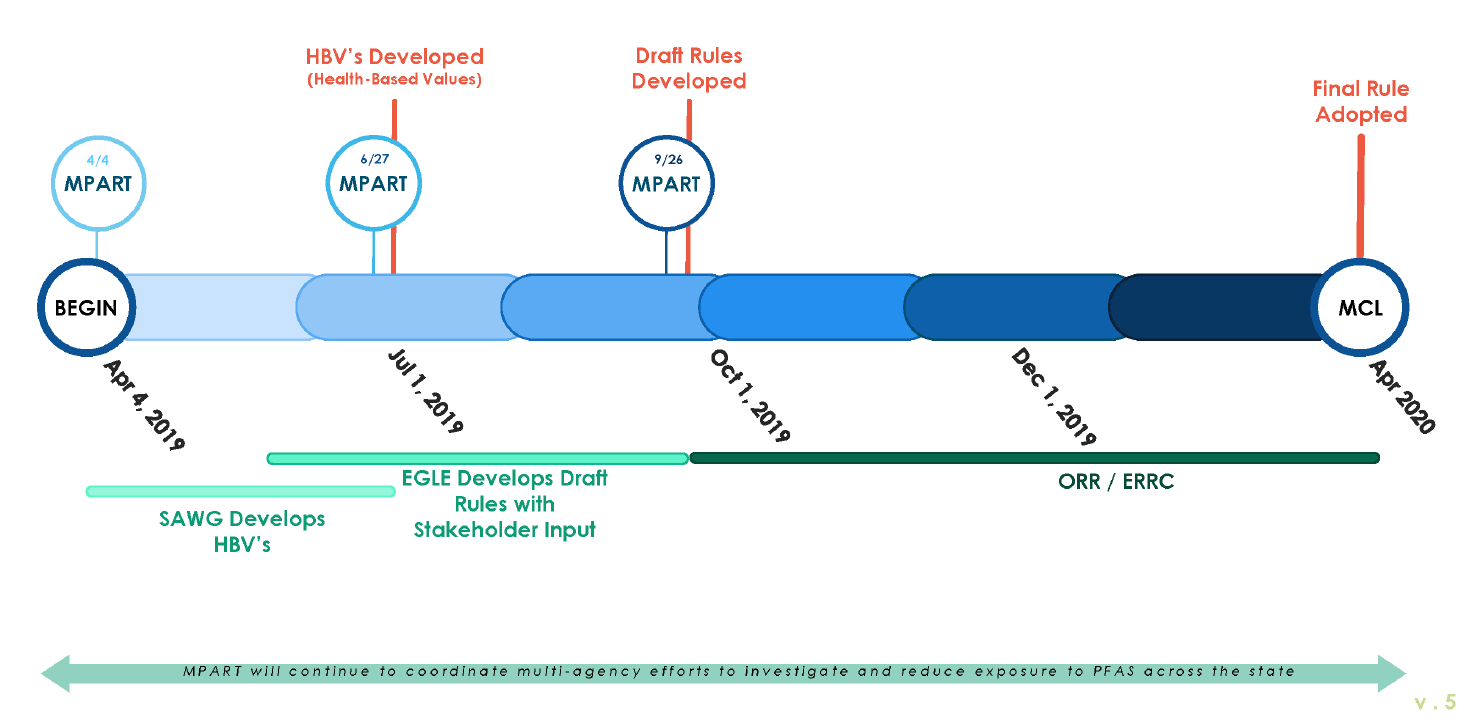
37
Appendix E: Timeline of the Maximum Contaminant Level Development Process
