BEST PRACTICES: FLUORIDE THERAPY
352 THE REFERENCE MANUAL OF PEDIATRIC DENTISTRY
Purpose
e American Academy of Pediatric Dentistry intends these
recommendations to help practitioners make decisions concern-
ing appropriate use of uoride as part of the comprehensive
oral health care for infants, children, adolescents, and persons
with special health care needs.
Methods
This document was initially developed by the Liaison with
Other Groups Committee, adopted in 1967
1
and last revised
by the Council on Clinical Affairs in 2018
2
. To update this
guidance, an electronic search of the PubMed
®
/MEDLINE
database was conducted using the terms: uoride caries pre-
vention, fluoridation, fluoride gel, fluoride varnish, fluoride
toothpaste, uoride therapy, silver diamine uoride, and topical
uoride; elds: all; limits: within last ve years, English. Be-
cause 4077 papers were identified through these electronic
searches, an alternate strategy of limiting the information
gathering to systematic review using the term uoride caries
prevention yielded 116 new systematic reviews or trials since
2017. Expert opinions and clinical practices also were relied
upon for these recommendations.
Background
Fluoride has been a major factor in the decline in prevalence
and severity of dental caries in the United States (U.S.) and
other economically developed countries. It has several caries-
protective mechanisms of action. Topically, low levels of
uoride in plaque and saliva inhibit the demineralization of
sound enamel and enhance the remineralization of demineralized
enamel.
3,4
e topical eect may be enhanced when combined
with good oral hygiene practices at home and use of a uoride
dentifrice.
5
Fluoride also inhibits dental caries by aecting the
metabolic activity of cariogenic bacteria.
6
High levels of uo-
ride, such as those attained with the use of topical gels or
varnishes, produce a temporary layer of calcium uoride-like
material on the enamel surface. e uoride is released when the
pH drops in response to acid production and becomes available
to remineralize enamel or aect bacterial metabolism.
7
Although
uoride-rich enamel is less acid-soluble than enamel with less
fluoride, the topical and remineralization effects of fluoride
have been found to have a greater impact on caries prevention
than incorporation of uoride into developing teeth.
8
Community water uoridation
Fluoridation of community drinking water is the most equitable
and cost-eective method of delivering uoride to all members
of most communities.
9
As of 2018, 73 percent of the U.S.
population on community water systems had access to uori-
dated water.
10
Water fluoridation at the level of 0.7-1.2
milligrams (mg) uoride ion per liter (i.e., parts per million
uoride [ppm F]) was introduced in the U.S. in the 1940s.
Since community water is now one of several sources of uoride,
ABBREVIATIONS
CaF: Calcium fluoride. F: Fluoride. FSIQ: Full scale intelligent quo-
tient. IQ: Intelligence quotient. mg: Milligrams. mg/kg: Milligrams
per kilogram. NaFV: Sodium fluoride varnish. ppm F: Parts per
million fluoride. SDF: Silver diamine fluoride. U.S.: United States.
Latest Revision
2023
Fluoride Therapy
How to Cite: American Academy of Pediatric Dentistry. Fluoride
therapy. The Reference Manual of Pediatric Dentistry. Chicago, Ill.:
American Academy of Pediatric Dentistry; 2023:352-8.
Abstract
This best practice provides information for practitioners regarding the use of fluoride as an aid in preventing and controlling dental caries in
pediatric dental patients. These recommendations address systemic fluoride (water fluoridation, dietary fluoride supplements), topical fluo-
ride delivery via professional application (acidulated phosphate fluoride gel or foam, sodium fluoride varnish, silver diamine fluoride), and
home-use products (toothpastes, mouthrinses) as well as the associated risks of fluoride agents. The standard level for community water
fluoridation (0.7 parts per million fluoride) helps balance the risk of caries and the possibility of dental fluorosis from excessive fluoride
ingestion during the early years of tooth development. Specific recommendations for dietary supplementation of fluoride for children ages
six months through 16 years are based on fluoride levels in the drinking water, other dietary sources of fluoride, use of a fluoridated tooth-
paste, and caries risk. The specific needs of each patient determine the appropriate use of systemic and topical fluoride products, whether
delivered in a professional clinical or a home setting. Fluoride has proven to be an effective therapy in reducing the prevalence of dental
caries in infants, children, adolescents, and persons with special needs.
Through a collaborative effort of the American Academy of Pediatric Dentistry Councils on Clinical Affairs and Scientific Affairs, this best
practice was revised to offer updated information and recommendations to assist healthcare practitioners and parents in using fluoride
therapy for management of caries risk in pediatric patients.
KEYWORDS: ADOLESCENT; CHILD; FLUORIDATION; FLUORIDE; ORAL HEALTH; SILVER DIAMINE FLUORIDE; TOOTHPASTE
BEST PRACTICES: FLUORIDE THERAPY
THE REFERENCE MANUAL OF PEDIATRIC DENTISTRY 353
the U.S. Department of Health and Human Services revised
these recommendations in 2015 to a standardized level of 0.7
ppm F to balance the benets of preventing dental caries while
reducing the chance of uorosis.
11
Community water uoridation has been associated with the
decline in caries prevalence in U.S. adolescents, from 90 per-
cent in at least one permanent tooth in 12-17-year-olds in the
1960s, to 60 percent in a 1999-2004 survey,
12,13
with more
recent estimates of 35 percent caries reduction in primary teeth
and 26 percent in permanent teeth of children
14
. Additionally,
a Cochrane review found that water uoridation led to a 15
percent increase in caries-free children in primary dentition and
14 percent increase in caries-free children with permanent
dentition.
14
Consuming fluoridated drinking water is both safe and
effective in preventing and controlling dental caries. Al-
though adverse health eects (e.g., decreased cognitive ability,
endocrine disruption, cancer) have been ascribed to the use of
uoride over the years, the preponderance of evidence from
large cohort studies and systematic reviews does not support an
association of such health issues and consumption of uoridated
water at the recommended concentration.
11
Regarding cognitive
ability, a recent study of mothers’ urinary uoride levels and
their child’s intelligence quotient (IQ) levels suggested an
association with exposure levels much greater than those rec-
ommended in the U.S. for water uoridation.
15
Also utilizing
maternal urinary fluoride levels, a multicenter prospective
cohort study
16
followed children born in Canada between
2008 and 2012. Forty-one percent of followed patients lived in
uoridated communities. is study assessed IQ at ages three
and four years using the Wechsler Preschool and Primary Scale
of Intelligence with Full Scale Intelligence Quotient (FSIQ) as
the primary outcome.
16
Results indicated that a one mg increase
in daily uoride intake (e.g., an extra six cups of optimally-
uoridated water each day) during pregnancy was associated
with a 4.49 point lower FSIQ score in boys but did not sig-
nicantly impact girls.
16
e study results suggested maternal
exposure to high uoride levels was associated with lower IQ
scores in boys and girls; however, it overlooked confounding
variables that did not adjust for dierences in socioeconomic
status or maternal IQ, and there was no IQ dierence when
evaluating the full population.
16
Moreover, a prospective study
in New Zealand did not support an association between uori-
dated water and IQ measurements
17
, and a national sample in
Sweden found no relationship between uoride levels in water
supplies and cognitive ability, noncognitive ability, and educa-
tion
18
. e current evidence does not support that consuming
water fluoridated at the level 0.7 ppm F is associated with
reductions in IQ.
Repeated consumption of fluoride at levels higher than
those recommended in this document during enamel devel-
opment, however, can cause dental uorosis (children 15-30
months of age being most susceptible for fluorosis of the
permanent incisors).
19
The National Health and Nutrition
Examination Survey (NHANES) 1999-2004 study found 23
percent of the U.S. population aged six through 49 had very
mild or mild uorosis.
20
Very mild and mild levels of uorosis
are associated with decreased caries experience and presents
clinically as an increase in diuse or lacy appearing white opaci-
ties of the enamel and generally are not considered an esthetic
problem.
21,22
e Iowa Fluoride Study was a longitudinal study
that gathered data on fluoride intake from multiple sources
(water, beverages, foods, uoride supplements, and dentifrices)
on subjects from birth to 36 months.
23
Those subjects were
examined at about age nine to assess permanent incisors and
rst molars for uorosis using the Fluorosis Risk Index.
24
is
study found the prevalence of mild uorosis was 13 percent
among those children with average uoride intakes of 0.04 mg
per kilogram (mg/kg) body weight and increased to 23 percent
when intakes were between 0.04 to 0.06 mg/kg.
24
When uo-
ride intakes average 0.06 mg/kg or more per day, mild uorosis
prevalence was 38 percent.
24
A more recent study found mild
uorosis levels increased to over 60 percentfor adolescents ages
16 and 17 in 2011-2012 compared to 29.4 percent in 2001-
2002; this is a greater than 31 percent increase.
25
Fluoride uoridation, supplements, and infant formula
Fluoride supplements are eective in reducing prevalence of
dental caries and may be considered for children at high caries
risk who drink fluoride-deficient (less than 0.6 ppm F) wa-
ter
26
(see Table). Fluoride supplementation schedules were last
revised in the early 1990s
27
and have not been adjusted since
1) uoride concentration in municipal water was standardized
and 2) recommendations to use fluoridated toothpaste with
the eruption of the rst tooth were promulgated.
Before prescribing supplements, determination of dietary
uoride intake from all sources can help reduce intake of ex-
cess uoride. Sources of dietary uoride may include drinking
water from home, day care, and school; beverages such as
soda
28
, juice
29
, and infant formula
30
; prepared food
31
; and
toothpaste. Concentrated infant formulas requiring reconsti-
tution with water have raised concerns regarding an increased
risk of uorosis.
32
Infants may be particularly susceptible be-
cause of the large consumption of such liquid while the body
weight is relatively low
4
and the enamel is mineralizing. An
evidence-based review found that consumption of reconstituted
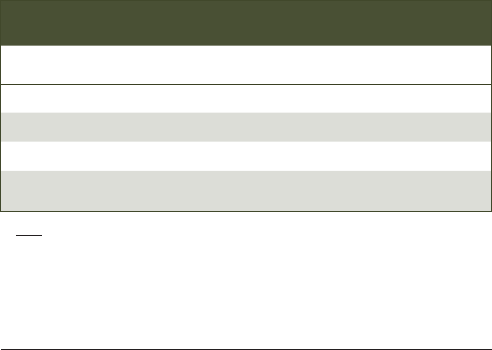
Table. DIETARY FLUORIDE SUPPLEMENTATION SCHEDULE
Age <0.3 ppm F 0.3 to 0.6 ppm F >0.6 ppm F
Birth to 6 months 0 0 0
6 months to 3 years 0.25 mg 0 0
3 to 6 years 0.50 mg 0.25 mg 0
6 to at least 16 years 1.00 mg 0.50 mg 0
Note: The recommendations in this table have not been revised since fluoride
concentration in municipal water was standardized and use of fluoridated
toothpaste for dentate infants was promulgated. All dietary sources of fluoride
should be taken into consideration before recommending fluoride supplements
for patients with fluoride-deficit community water.

BEST PRACTICES: FLUORIDE THERAPY
354 THE REFERENCE MANUAL OF PEDIATRIC DENTISTRY
infant formula can be associated with an increased risk of mild
uorosis but recommended the continued use of uoridated
water.
33
One study has shown that dental uorosis levels do
not vary in fluoridated areas regardless of premixed versus
reconstituted formula.
34
Nevertheless, over-supplementation of
uoride, even for patients residing in areas with unuoridated
water, can cause uorosis.
35
Since standardization of the optimal
uoride levels in drinking water to 0.7 ppm F in 2015, dental
uorosis is less likely to occur. However, caution is indicated
when considering the use of uoride supplements for children
under age six due to their continued dental development and
consumption of uoride from a variety of sources.
Professionally-applied uoride varnish, gel, and foam
Professionally-applied topical uoride treatments are ecacious
in reducing prevalence of dental caries. e most commonly
used agents for professionally-applied uoride treatments are
five percent sodium fluoride varnish ([NaFV]; 2.26 percent
uoride [F], 22,600 ppm F) and acidulated phosphate uoride
([APF]; 1.23 percent F, 12,300 ppm F). Meta-analyses of 23
clinical trials, most with twice yearly application, favors the use
of uoride varnish in primary and permanent teeth to prevent
decay.
36
Fluoride varnish appears to be eective at preventing
caries in higher-risk children younger than ve years of age.
37
Unit doses of ve percent uoride varnish are the only pro-
fessional topical fluoride agent recommended for children
younger than age six for safety reasons.
36
Meta-analyses of
placebo-controlled trials show that fluoride gels, applied at
three-months to one-year intervals, also are ecacious in re-
ducing caries in permanent teeth.
38,39
Some topical uoride gel
and foam products are marketed with recommended treatment
times of less than four minutes, but there are no clinical trials
showing efficacy of shorter than four-minute application
times.
40
Evidence that topical uoride foams are ecacious in
children is limited.
36
Children at risk for caries should receive
a professional uoride treatment at least every six months.
40
In
2014, the U.S. Preventive Services Task Force recommended
a schedule for uoride varnish application specically by non-
dental personnel to provide this preventive strategy to children
in medical settings, especially when children are more likely to
see a medical provider rather than a dental provider.
41,42
Recent
meta-analyses tried to determine whether professionally-applied
uoride can reverse incipient/white spot caries lesions
43-45
but,
due to heterogeneity of studies included in the systematic
review coupled with home use of uoride dentifrices by research
subjects, a valid conclusion could not be made
43
. Yet another
study has shown that incipient enamel lesions (International
Caries Detection and Assessment System Code 2) can be
arrested with semiannual applications of ve percent NaFV.
46
Silver diamine uoride
irty-eight percent silver diamine uoride ([SDF]; ve percent
F, 44,800 ppm F) has been cleared by the U.S. Food and Drug
Administration as a dentin desensitizer in adults.
47
It currently
is used frequently to arrest cavitated caries lesions. SDF is
thought to arrest caries by the antibacterial eect of silver and
remineralization of enamel and dentin by fluoride.
48
Silver
ions have an antimicrobial eect mainly in the treated carious
dentin
49
, and the combination of silver and uoride in an alka-
line solution have a synergistic eect that creates an unfavorable
environment for collagen enzyme activation, thereby reducing
dentin degradation.
50
Clinical trials show caries arrest rates
ranging from 35 to 80 percent
51
, but such studies have a high
risk of bias and a high heterogeneity between them, leading to
conditional recommendations for its use.
52
Numerous clinical
trials conclude that biannual application of SDF results in
higher caries arrest in dentin caries lesions as compared to uoride
varnish.
47.53
us, SDF is an important adjunct therapy in the
individualized comprehensive care plan for children and adoles-
cents for whom access to denitive dental restorative care may
be limited for a variety of reasons or preferentially postponed.
As the product is highly concentrated, less than a drop is
needed to treat several caries lesions, making it cost-eective.
SDF is best used as part of an ongoing caries management
plan within the context of a dental home.
54,55
SDF is safe to use in children and adults when delivered in
accordance with dosing and application criteria.
56
While current
data on the systemic eects of silver is limited
47
, data supports
a cytotoxic eect to the dental pulp cells when applied directly
on pulp tissue
57-59
. SDF solution, when applied to deep caries
lesions (0.25-0.5 millimeters dentin thickness remaining), can
be rapidly absorbed into dentin and produce a mild inam-
mation.
60
Whether tertiary dentin formation is a response to
cariogenic bacteria or to the SDF remains undetermined.
60
Two investigations
61,62
have evaluated SDF as an indirect pulp
therapy medicament. One study
62
found application of SDF
arrested further caries progression but did not significantly
increase the amount of reparative dentin radiographically.
Similarly, the other found no significant difference between
SDF, SDF combined with potassium iodide, and the control
(resin-modied glass ionomer) at preventing secondary caries.
61
e absence of postoperative pain and maintenance of tooth
vitality indicated that SDF did not adversely aect the pulp
when applied as an indirect pulp therapy agent.
61
e other
reported side eects of SDF are that caries lesions stain black
after treatment and skin and gingiva temporarily stain with
contact.
Home-use uoride products
The goal of home-use fluoride products for children is to
maximize the time uoride is in direct contact with the tooth
surface, in lower-dose higher-frequency approaches.
63
In chil-
dren having higher baseline levels of caries, utilizing higher
concentrations of fluoride in the toothpaste, brushing with
greater frequency, and having supervision of brushing were
ecacious in reducing the prevalence of dental caries in perma-
nent teeth.
64,65
A meta-analysis of eight clinical trials on caries
increment in preschool children also shows that toothbrushing
with uoridated toothpaste signicantly reduces dental caries
prevalence in the primary dentition.
66
Using no more than a

BEST PRACTICES: FLUORIDE THERAPY
THE REFERENCE MANUAL OF PEDIATRIC DENTISTRY 355
smear or rice-sized amount (0.1 mg F) of uoridated toothpaste
for children less than three years of age may decrease risk of
uorosis. Using no more than a pea-sized amount (0.25 mg F)
of uoridated toothpaste is appropriate for children aged three
to six
67.68
(see Figure). To maximize the beneficial effect of
fluoride in the toothpaste, supervised toothbrushing should
be done twice a day, and rinsing after brushing should be kept
to a minimum or avoided altogether.
69
Other topical uoride
products (e.g., prescription-strength home-use 0.5 percent F
gels and pastes; prescription-strength home-use 0.09 percent
F mouthrinse) have benet in reducing dental caries in those
patients at higher risk, such as adolescents, adolescents with
special health care needs, or patients with xed orthodontic
appliances; these products are recommended for use in chil-
dren six years or older.
36
Having children spit after brushing
and parents supervise the amounts administered to children
will help avoid over-ingestion. Over-ingestion of uoridated
toothpaste combined with other dietary uoride sources may
lead to daily intake greater than the recommended amount
and could lead to development of dental uorosis.
4
Over 20,000 reports per year regarding uoride ingestion
are received at poison control centers
70
, and over 80 percent of
suspected cases occur in the under-six-years age group
71
. e
probably-toxic dose for uoride is ve mg/kg body weight.
72
Lower dosage may result in gastrointestinal disturbances with
higher doses producing central nervous system side eects such
as seizures or tetany.
73
Fifteen mg/kg body weight of uoride
likely could be fatal for a small child.
74
Over-the-counter
toothpastes approved by the American Dental Association con-
tain at least 1000 ppm F and less than 1500 ppm F.
75
Currently
available prescription strength toothpastes may contain 5000
ppm F
75
or 605 mg F per 100 milliliters
76
. Parental dispensing
of toothpaste for use by children under the age of three,
supervised toothbrushing for all children unable to expectorate,
and keeping prescription uoride supplements and/or home-
use fluoride products out of reach of young children can
prevent unintended ingestion which has acute (toxicity) as
well as chronic (uorosis) implications.
Recommendations
e AAPD recommends:
1. the use of uoride for the prevention and control of
caries as it is both safe and highly eective in reducing
dental caries prevalence.
2. consumption of optimally-fluoridated community
water as a cost-eective method to prevent and control
caries at the population level.
3. toothbrushing at least twice daily with an age-
appropriate amount of over-the-counter fluoride-
containing toothpaste to prevent caries as rst line for
caries prevention.
4. professionally-applied topical uoride treatments such
as ve percent NaFV or 1.23 percent F gel preparations
at least twice per year to reduce incidence of dental caries.
5. 38 percent SDF be used to arrest cavitated caries le-
sions in primary teeth and permanent teeth as part of a
comprehensive caries management program.
6. prescription-strength home-use 0.5 percent F gels and
pastes and 0.02-0.09 percent F mouth rinses to reduce
dental caries in high-risk patients over six years of age.
7. decisions concerning the administration of uoride be
based on the unique needs of each patient, including
the risks and benets (e.g., risk of mild or moderate
uorosis versus the benets of decreasing caries incre-
ment and, in some cases, preventing devastating dental
disease).
8. uoride dietary supplements be cautiously considered
for children at caries risk who drink less than optimally-
fluoridated water as supplementation, in the face of
all other sources of uoride, could exceed the recom-
mended amount of daily uoride intake.
References
1. American Academy of Pedodontics. Fluoride. Chicago, Ill.
1967.
2. American Academy of Pediatric Dentistry. Fluoride therapy.
Pediatr Dent 2018;40(6):250-3.
3. Featherstone JD. Prevention and reversal of dental caries:
Role of low level fluoride. Community Dent Oral
Epidemiol 1999;27(1):31-40.
4. Tinano N. Use of uoride. In: Berg J, Slayton RA, eds.
Early Childhood Oral Health. 2nd ed. Hoboken, N.J.:
Wiley-Blackwell; 2016:1064.
5. Toumba KJ, Twetman S, Splieth C, Parnell C, van Loveren
C, Lygidakis NA. Guidelines on the use of uoride for
caries prevention in children: An updated EAPD policy
document. Euro Arch Paed Dent 2019;20:507-16.
6. Buzalaf MA, Pessan JP, Honório HM, ten Cate JM.
Mechanism of action of fluoride for caries control.
Monogr Oral Sci 2011;22:97-114.
7. Center for Disease Control and Prevention. Recommen-
dations for using uoride to prevent and control dental
caries in the United States. MMWR Recomm Rep 2001;
50(RR-14):1-42.
Figure. Comparison of a smear (left) with a pea-sized (right) amount
of toothpaste.
References continued on the next page.

BEST PRACTICES: FLUORIDE THERAPY
356 THE REFERENCE MANUAL OF PEDIATRIC DENTISTRY
8. Gerth HU, Dammaschke T, Schäfer E, Züchner H. A
three-layer structure model of fluoridated enamel con-
taining CaF
2
, Ca(OH)
2
and FAp. Dent Mater 2007;23
(12):1521-8.
9. Division of Oral Health, National Center for Chronic
Disease Prevention and Health Promotion, Center for
Disease Control and Prevention. Achievements in public
health, 1900-1999; Fluoridation of drinking water to
prevent dental caries. JAMA 2000;283(10):1283-6.
10. Centers for Disease Control and Prevention. Division of
Oral Heath, National Center for Chronic Disease Prevention
and Health Promotion. Community Water Fluoridation:
Water Fluoridation Data & Statistics. Last reviewed: August
28, 2020. Available at: “https://www.cdc.gov/uoridation/
statistics/index.htm”. Accessed May 24, 2023.
11. U.S. Department of Health and Human Services Panel
on Community Water Fluoridation. U.S. Public Health
Services recommendation for fluoride concentration in
drinking water for the prevention of dental caries. Public
Health Reports 2015;130(4):318-31. Available at: “https:
//www.ncbi.nlm.nih.gov/pmc/articles/PMC4547570/”.
Accessed May 24, 2023.
12. U.S. Department of Health and Human Services. Proposed
HHS recommendation for fluoride concentration in
drinking water for prevention of dental caries. Federal
Register 2011;76(9):2383-8. Available at: “https://www.
govinfo.gov/content/pkg/FR-2011-01-13/pdf/2011-637.
pdf”. Accessed May 24, 2023.
13. Dye BA, Tan S, Smith V, et al. Trends in oral health
status: United States, 1988-1994 and 1999-2004. Vital
Health Stat 2007;11(248):1-92. Available at: “https://
www.cdc.gov/nchs/data/series/sr_11/sr11_248.pdf”.
Accessed May 24, 2023.
14. Iheozor-Ejiofor A, Worthington HV, Walsh T, et al. Water
uoridation for the prevention of dental caries. Cochrane
Database Syst Rev 2015;(6):CD010856. Available at:
“https://www.cochranelibrary.com/cdsr/doi/10.1002/
14651858.CD010856.pub2/pdf/full”. Accessed January
3, 2023.
15. Bashash M, Thomas D, Hu H, et al. Prenatal fluoride
exposure and cognitive outcomes in children at 4 and
6-12 years of age in Mexico. Environ Health Perspect
2017;125(9):097017. Available at: “https://www.ncbi.
nlm.nih.gov/pmc/articles/PMC5915186/”. Accessed
November 3, 2022.
16. Green R, Lanphear B, Hornung R, et al. Association be-
tween maternal uoride exposure during pregnancy and
IQ scores in ospring in Canada. JAMA Pediatr 2019;
173(10):940-8. Available at: “https://jamanetwork.com/
journals/jamapediatrics/article-abstract/2748634”.
Accessed January 1, 2023.
17. Broadbent JM, omson WM, Ramrakha S, et al. Com-
munity water uoridation and intelligence: Prospective
study in New Zealand. Am J Public Health 2015;105(1):
72-6.
18. Aggeborn L, Öhman M. e eects of uoride in drink-
ing water. 2017. Available at: “https://www.ifau.se/
globalassets/pdf/se/2017/wp2017-20-the-eects-of-uoride-
in-the-drinking-water.pdf”. Accessed June 6, 2023.
19. Evans RW, Darvell BW. Refining the estimate of the
critical period for susceptibility to enamel fluorosis in
human maxillary central incisors. J Pub Health Dent
1995;55(4):238-49.
20. Beltrán-Aguilar ED, Barker L, Dye BA. Prevalence and
severity of dental fluorosis in the United States, 1999-
2004. NCHS Data Brief, No. 53. Hyattsville, Md.:
National Center for Health Statistics. 2010:1-8. Available
at: “https://www.cdc.gov/nchs/data/databriefs/db53.pdf”.
Accessed May 24, 2023.
21. Chankanka O, Levy SM, Warren JJ, Chalmers JM. A lit-
erature review of aesthetic perceptions of dental uorosis
and relationships with psychosocial aspects/oral health-
related quality of life. Community Dent Oral Epidemiol
2010;38(2):97-109.
22. Szpunar SM, Burt BA. Dental caries, uorosis, and uoride
exposure in Michigan schoolchildren. J Dent Res 1988;
67(5):802-6.
23. Levy SM, Warren JJ, Davis CS, Kirchner HL, Kanellis
MJ, Wefel JS. Patterns of uoride intake from birth to
36 months. J Public Health Dent 2001;61(2):70-7.
24. Hong L, Levy SM, Warren JJ, et al. Fluoride intake levels
in relation to uorosis development in permanent maxil-
lary central incisors and rst molars. Caries Res 2006;40
(6):494-500.
25. Wiener RC, Shen C, Findley P, Tan X, Sambamoorthi U.
Dental fluorosis over time: A comparison of National
Health and Nutrition Examination Survey data from
2001-2002 and 2011-2012. J Dent Hyg 2018;92(1):23-9.
Available at: “https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC5929463/”. Accessed March 10, 2023.
26. Rozier RG, Adair S, Graham F, et al. Evidence-based
clinical recommendations on the prescription of dietary
fluoride supplements for caries prevention: A report of
the American Dental Association Council on Scientic
Aairs. J Am Dent Assoc 2010;141(12):1480-9.
27. Adair SM. Overview of the history and current status of
uoride supplementation schedules. J Pub Health Dent
1999;59(4):252-8.
28. Heilman JR, Kiritsy MC, Levy SM, Wefel JS. Assessing
fluoride levels of carbonated soft drinks. J Am Dent
Assoc 1999;130(11):1593-9.
29. Kiritsy MC, Levy SM, Warren JJ, Guha-Chowdhury N,
Heilman JR, Marshall T. Assessing fluoride concentra-
tions of juices and juice-flavored drinks. J Am Dent
Assoc 1996;127(7):895-902.
30. Levy SM, Kohout FJ, Guha-Chowdhury N, Kiritsy MC,
Heilman JR, Wefel JS. Infants’ fluoride intake from
drinking water alone, and from water added to formula,
beverages, and food. J Dent Res 1995;74(7):1399-407.

BEST PRACTICES: FLUORIDE THERAPY
THE REFERENCE MANUAL OF PEDIATRIC DENTISTRY 357
31. Heilman JR, Kiritsy MC, Levy SM, Wefel JS. Fluoride
concentrations of infant foods. J Am Dent Assoc 1997;
128(7):857-63.
32. Hujoel PP, Zina LG, Moimas SAS, Cunha-Cruz J. Infant
formula and enamel uorosis. A systematic review. J Am
Dent Assoc 2009;140(7):841-54.
33. Berg J, Gerweck C, Hujoel PP, et al. Evidence-based
clinical recommendations regarding uoride intake from
reconstituted infant formula and enamel uorosis. J Am
Dent Assoc 2011;142(1):79-87.
34. Do LG, Levy SM, Spencer AJ. Association between infant
formula feeding and dental fluorosis and caries in
Australian children. J Public Health Dent 2012;72(2):
112-21.
35. Pendrys DG. Risk of enamel uorosis in nonuoridated
and optimally uoridated populations: Considerations for
the dental professional. J Am Dent Assoc 2000;131(6):
746-55.
36. Weyant RJ, Tracy SL, Anselmo T, et al. Topical uoride
for caries prevention: Executive summary of the updat-
ed clinical recommendations and supporting systematic
review. J Amer Dent Assoc 2013;144(11):1279-91. Avail-
able at: “https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC4581720/”. Accessed January 1, 2023.
37. Chou R, Pappas M, Dana T, et al. Screening and Interven-
tions to Prevent Dental Caries in Children Younger an
Age Five Years: A Systematic Review for the U.S. Pre-
ventive Services Task Force. Evidence Synthesis No. 210.
AHRQ Publication No. 21-05279-EF-1. Rockville, Md.:
Agency for Healthcare Research and Quality; 2021:15-18.
Available at: “https://www.ncbi.nlm.nih.gov/books/
NBK575915/”. Accessed June 6, 2023.
38. Marinho VCC. Cochrane reviews of randomized trials of
uoride therapies for preventing dental caries. Euro Arch
Paed Dent 2009;10(3):183-91.
39. Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride
toothpaste for preventing dental caries in children and
adolescents. Cochrane Database Syst Rev 2003;(1):
CD002278. Available at: “ncbi.nlm.nih.gov/pmc/articles/
PMC8439270/”. Accessed May 24, 2023.
40. Hunter JW, Chan JT, Featherstone DB, et al.
Professionally-applied topical fluoride: Evidence-based
clinical recommendations. J Am Dent Assoc 2006;137
(8):1151-9.
41. Moyer VA, U.S. Preventive Services Task Force. Prevention
of dental caries in children from birth through age 5
years: U.S. Preventive Services Task Force recommendation
statement.Pediatrics 2014;133(6):1102-11. Available at:
“https://publications.aap.org/pediatrics/article-abstract/
133/6/1102/76111/Prevention-of-Dental-Caries-in-
Children-From-Birth?redirectedFrom=fulltext”. Accessed
March 1, 2023.
42. U.S. Preventive Services Task Force; Davidson KW, Barry
MJ, et al. Screening and interventions to prevent dental
caries in children younger than 5 years: U.S. Preventive
Services Task Force recommendation statement. JAMA
2021;326(21):2172-8. Available at: “https://jamanetwork.
com/journals/jama/fullarticle/2786823”. Accessed May
24, 2023.
43. Cumerlato CBdF, Santos CSD, Rotta RN, Cademartori
MG, Corrêa MB. Is professionally applied topical uoride
eective in treating incipient caries? A systematic review.
Braz Oral Res 2022;36:e083.
44. Gao SS, Zhang S, Mei ML, Lo ECM, Chu CH. Caries
remineralization and arresting eect in children by profes-
sionally applied uoride treatment. A systematic review.
BMC Oral Health 2016;16:12. Available at: “https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC4736084/pdf
/12903_2016_Article_171.pdf”. Accessed February 26,
2023.
45. Lenzi TL, Montagner AF, Soares FZ, de Oliveira Rocha
R. Are topical fluorides effective for treating incipient
carious lesions?: A systematic review and meta-analysis. J
Am Dent Assoc 2016;147(2):84-92.
46. Phonghanyudh A, Duangthip D, Mabangkhru S,
Jirarattanasopha V. Is silver diamine uoride eective in
arresting enamel caries? A randomized clinical trial.Int J
Environ Res Public Health 2022;19(15):8992. Available
at: “https://www.ncbi.nlm.nih.gov/pmc/articles/PMC
9331268/”. Accessed March 12, 2023.
47. Crystal YO, Niederman R. Evidence-based dentistry
update on silver diamine uoride. Dent Clin N Am 2019;
63(1):45-68.
48. Zhao IS, Gao SS, Hiraishi N, et al. Mechanisms of silver
diamine uoride on arresting caries: A literature review.
Int Dent J 2018;68(2):67-76.
49. Sulyanto RM, Kang M, Srirangapeatanam S, Berger M, et
al. Biomineralization of dental tissues treated with silver
diamine uoride. J Dent Res 2021;100(10):1099-108.
50. Mei ML, Lo ECM, Chu CH. Arresting dentine caries
with silver diamine fluoride: What’s behind it? J Dent
Res 2018;97(7):751-8.
51. Gao SS, Zhao IS, Hiraishi N, et al. Clinical trials of silver
diamine fluoride in arresting caries among children: A
systematic review. JDR Clin Trans Res 2016;1(3):
201-10.
52. Crystal YO, Marghalani AA, Ureles SD, et al. Use of
silver diamine uoride for dental caries management in
children and adolescents, including those with special
health care needs. Pediatr Dent 2017;39(5):E135-E145.
53. Mabangkhru S, Duangthip D, Hung CC, Phonghanyudh
A, Jirarattanasopha V. A randomized clinical trial to arrest
dentin caries in young children using silver diamine
uoride. J Dent 2020;99:103375.
54. American Academy of Pediatric Dentistry. Policy on the
use of silver diamine uoride for pediatric dental patients.
e Reference Manual of Pediatric Dentistry. Chicago,
Ill.: American Academy of Pediatric Dentistry; 2023:
103-5.
References continued on the next page.

BEST PRACTICES: FLUORIDE THERAPY
358 THE REFERENCE MANUAL OF PEDIATRIC DENTISTRY
55. Krol DM, Whelan K, AAP Section on Oral Health.
Maintaining and improving the oral health of young
children. Pediatrics 2023;151(1):e2022060417. Available
at: “https://doi.org/10.1542/peds.2022-060417”. Accessed
May 24, 2023.
56. American Academy of Pediatric Dentistry. Chairside
guide: Silver diamine fluoride in the management of
dental caries lesion. e Reference Manual of Pediatric
Dentistry. Chicago, Ill.: American Academy of Pediatric
Dentistry; 2023:638-9.
57. Manuschai J, Talungchit S, Naorungroj S. Penetration of
silver diamine uoride in deep carious lesions of human
permanent teeth: An in vitro study. Int J Dent 2021;2021:
3059129. Available at: “https://www.ncbi.nlm.nih.gov/
pmc/articles/PMC8716243/”. Accessed May 24, 2023.
58. Srisomboon S, Kettratad M, Stray A, et al. Eects of silver
diamine nitrate and silver diamine fluoride on dentin
remineralization and cytotoxicity to dental pulp cells: An
in vitro study. J Funct Biomater 2022;13(1):16-28.
59. Zaeneldin A, Yu OY, Chu CH. Eect of silver diamine
uoride on vital dental pulp: A systematic review. J Dent
2022;119:104066. Available at: “https://doi.org/10.10
16/j.jdent.2022.104066”. Accessed May 24, 2023.
60. Korwar A, Sharma S, Logani A, Shah N. Pulp response
to high fluoride releasing glass ionomer, silver diamine
fluoride, and calcium hydroxide used for indirect pulp
treatment: An in vivo comparative study. Contemp Clin
Dent 2015;6(3):288-92.
61. Baraka M, Tkeya M, Bakry NS, Fontana M. Twelve-
month randomized controlled trial of 38% silver diamine
fluoride with or without potassium iodide in indirect
pulp capping of young permanent molars. J Am Dent
Assoc 2022;153(12):1121-33.
62. Divyashree R. Effectiveness of silver diamine fluoride
when used as an indirect pulp therapy (IPT) material—A
clinical and radiographic assessment. Int J Applied Dent
Sci 2021;7(2):466-78. Available at: “https://doi.org/10.
22271/oral.2021.v7.i2g.1255”. Accessed January 24, 2023.
63. Adair SM. Evidence-based use of fluoride in contem-
porary pediatric dental practice. Pediatr Dent 2006;28
(2):133-42.
64. Marinho VCC, Higgin JP, Logan, S, Sheiham A.
Systematic review of controlled trials on the eectiveness
of fluoride gels for the prevention of dental caries in
children. J Dent Educ 2003;67(4):448-58.
65. Walsh T, Worthington HV, Glenny AM, Marinho VCC,
Jeroncic A. Fluoride toothpastes of dierent concentra-
tions for preventing dental caries. Cochrane Database
Syst Rev 2019;(1):CD007868. Available at: “https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC6398117/”. Accessed
June 6, 2023.
66. Dos Santos APP, Nadanovsky P, Oliveira BH. A systematic
review and meta-analysis of the eects of uoride tooth-
paste on the prevention of dental caries in the primary
dentition of preschool children. Community Dent Oral
Epidemiol 2013;41(1):1-12.
67. American Dental Association Council on Scientific
Aairs. Fluoride toothpaste use for young children. J Am
Dental Assoc 2014;145(2):190-1.
68. Wright JT, Hanson N, Ristic H, et al. Fluoride toothpaste
efficacy and safety in children younger than 6 years. J
Am Dent Assoc 2014;145(2):182-9.
69. Scottish Intercollegiate Guidelines Network (SIGN).
Dental interventions to prevent caries in children. March
2014. Edinburgh: SIGN; 2014. (SIGN publication no.
138). Available at: “https://www.scottishdental.org/wp
-content/uploads/2014/04/SIGN138.pdf”. Accessed June
6, 2023.
70. Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL,
Rumack BH, Giffin SL. 2009 Annual Report of the
American Association of Poison Control Centers’ National
Poison Data System (NPDS): 27th Annual Report. Clin
Toxicol (Phila) 2010;48(10):979-1178. Erratum in: Clin
Toxicol (Phila) 2014;52(10):1284. Available at: “https://
www.tandfonline.com/doi/full/10.3109/15563650.2010.
543906”. Accessed May 24, 2023.
71. Shulman JD, Wells LM. Acute fluoride toxicity from
ingesting home-use dental products in children, birth to
6 years of age. J Pub Health Dent 1997;57(3):150-8.
72. Whitford GM. Fluoride in dental products: Safety
considerations. J Dent Res 1987;66(5):1056-60.
73. Smith FA.Fluoride toxicity. In: Corn M, ed. Handbook
of Hazardous Materials. New York, N.Y.: Academic Press;
1993:277-83.
74. Whitford GM. Acute toxicity of ingested fluoride.
Monog Oral Sci 2011;22:66-80.
75. American Dental Association. Oral Health Topics. Fluo-
ride: Topical and systemic supplements. 2021. Available
at: “https://www.ada.org/resources/research/science-and
-research-institute/oral-health-topics/uoride-topical-and
-systemic-supplements”. Accessed March 12, 2023.
76. Drugs.com. Sodium uoride paste prescribing informa-
tion. Available at: “https://www.drugs.com/pro/sodium
-uoride-paste.html”. Accessed March 12, 2023.
