

State of Hawaiʻi
Department of Business, Economic Development, and Tourism
Hawaiʻi State Energy Office
CLEAN ENERGY EDUCATION CURRICULA and TOOLKITS
The purpose of the Hawaiʻi State Energy Office (HSEO) is to promote energy efficiency, renewable
energy, and clean transportation to help achieve a resilient clean energy economy by 2045. HSEO is
developing a statewide clean energy public education and outreach program to empower teachers’,
students’, and their families’ participation in Hawaiʻi ’s transition to a decarbonized economy; and to
encourage Hawaiʻi ’s K-12 students to become the next generation of clean energy leaders.
This project and material is based upon work supported by the U.S. Department of Energy, Office of
Energy Efficiency and Renewable Energy (EERE) under the State Energy Program Award Number
DE-EE0008286.
This report was prepared as an account of work sponsored by an agency of the United States
Government. Neither the United States Government nor any agency thereof, nor any of their
employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for
the accuracy, completeness, or usefulness of any information, apparatus, product, or process
disclosed, or represents that its use would not infringe privately owned rights. Reference herein to
any specific commercial product, process, or service by trade name, trademark, manufacturer, or
otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by
the United States Government or any agency thereof. The views and opinions of authors expressed
herein do not necessarily state or reflect those of the United States Government or any agency
thereof.
1

Developed by Maui Economic Development Board, STEMworks
TM
on contract for the
Hawaiʻi State Energy Office
Curriculum at a Glance
Grade Levels
6 - 8
Time Required
7 Class Periods
Activity Group Sizes
3 - 4
NGSS Performance
Expectations
MS-PS1-2, MS-PS1-5, MS-PS1-6, Oxidation/Reduction (redox),
MS-ETS1-1, MS-ESS3-3,
Activity Title
Guiding Questions
Activity: Electricity
Exploration
What items need electricity to work?
Where does electrical energy come from?
Scientific Research:
Hydroelectricity
What is hydroelectricity?
What forms of hydroelectricity do we have in Hawaii?
How else could the ocean be used to generate electrical energy?
Kit Discovery:
Electrochemistry, Metals,
and the Impacts of Mining
What is included in my kit?
Where do the metals and chemicals in this kit come from?
What can they be used for?
What impacts does mining raw materials have on the environment?
Lab 1: Aluminum Eater -
Chemical Reactions
What is a chemical reaction?
What observations tell me a chemical reaction has occurred?
Lab 2: Voltage Tests
How does a voltmeter work and what does it measure?
What materials can I use to create a circuit?
Which metals would I choose to power an electronic alarm clock?
Where do metals come from?
How does metal extraction and mining impact the environment?
Lab 3: Coin Cell Battery
Flashlight
What is a battery and how does it work?
How is chemical energy from a battery transformed into electricity?
Lab 4: Saltwater Powered
Clock
How are chemistry and electricity related?
2
At Home Activities:
1. Energy Consumption
Monitoring
2. Cookie Mining &
Reclamation
What items in my home need electricity to work?
How do I read an electric bill?
How can I reduce energy consumption? Test this for one month!
What impacts does mining have on the earth?
What is reclamation and is it easy to do?
Summary
What is energy? Energy is all around us. It is the power that brings nature, humans and machines to
life. The purpose of this renewable energy curriculum is to provide learners in grades 6-8 with the
opportunity to begin building energy literacy through exploration, experimentation, engineering, and
hands-on fun. In the pages that follow, you and your students will explore the relationship between
chemistry and electricity through experimentation. Students will begin with a laboratory activity to
build their conceptual understanding of chemical reactions, learn how to use a voltmeter to test the
voltage potential of different metals, build a simple coin cell battery to power an LED light, and
discover the role that saltwater could play in our renewable energy future. Throughout these
hands-on activities, students will also be presented with fun facts, key renewable energy concepts,
career tips, and more.
The classroom toolkit for this curriculum includes a book on hydroelectricity to help build literacy and
critical thinking skills. Hydroelectricity is a form of alternative energy that uses the motion of water via
gravity, tides, and waves to create electricity. Here in Hawaii, the ocean is a tremendous, potential
source of energy. The hands-on chemistry experiments will challenge students to think more
creatively about how ocean water could be used to create electricity beyond waves, tide, and ocean
thermal energy conversion. In each of the activities and four hands-on labs, students will make
hypotheses, collect data through observations, analyze their experimental results, engineer a light
and a clock and troubleshoot their constructions as needed - just like real scientists and engineers.
We have also included several online extension activities that can be done in the classroom or at
home, as well as an at home extension activity on home electricity usage to engage other members
of the household. This renewable energy curriculum and the inquiry-based labs are aligned to both
Physical Science and Engineering Next Generation Science Standards (NGSS).
--------------------------------------------------------------------------------------------------------------------------------------
Growth Objectives: Science and Engineering Practices
Developing and Using Models: A practice of both science and engineering is to use and construct
models as helpful tools for representing ideas and explanations. These tools include diagrams,
drawings, physical replicas, mathematical representations, analogies, and computer simulations.
Modeling tools are used to develop questions, predictions and explanations; analyze and identify
flaws in systems; and communicate ideas. Models are used to build and revise scientific explanations
and proposed engineered systems. Measurements and observations are used to revise models and
designs.
3

● Develop and/or use a model to generate data to test ideas about phenomena in natural or
designed systems, including those representing inputs and outputs, and those at unobservable
scales. Students will develop models to generate data to test ideas about chemical reactions,
the conservation of matter, voltages, the transfer of chemical energy to electrical energy and
the flow of electricity.
Planning and Carrying Out Investigations: Scientists and engineers plan and carry out
investigations in the field or laboratory, working collaboratively as well as individually. Their
investigations are systematic and require clarifying what counts as data and identifying variables or
parameters. Engineering investigations identify the effectiveness, efficiency, and durability of
designs under different conditions.
● Conduct an investigation and/or evaluate and/or revise the experimental design to produce
data to serve as the basis for evidence that meet the goals of the investigation. In all four labs,
students will need to work together to investigate the phenomena in question for each
experiment, and evaluate if they met the goals of the investigation. If a circuit does not perform
as required, they have not met the goals of their investigation. In this case, they’ll need to work
together as a team to troubleshoot their configurations.
● Collect data about the performance of a proposed object, tool, process, or system under a
range of conditions. In Lab 2, students will collect data to test the various voltages of different
metal combinations (electrical potential).
Constructing Explanations and Designing Solutions: The end-products of science are
explanations and the end-products of engineering are solutions. The goal of science is the
construction of theories that provide explanatory accounts of the world. A theory becomes accepted
when it has multiple lines of empirical evidence and greater explanatory power of phenomena than
previous theories. The goal of engineering design is to find a systematic solution to problems that is
based on scientific knowledge and models of the material world. Each proposed solution results from
a process of balancing competing criteria of desired functions, technical feasibility, cost, safety,
aesthetics, and compliance with legal requirements. The optimal choice depends on how well the
proposed solutions meet criteria and constraints.
● Apply scientific ideas or principles to design, construct, and/or test a design of an object, tool,
process or system. Students will use the scientific information they learned about chemical
reactions, electricity, and circuits in Labs 1 and 2 to build functional, electrical products in Labs
3 and 4.
● Optimize performance of a design by prioritizing criteria, making tradeoffs, testing, revising,
and retesting In Labs 3 - 4, students will build their own battery to power a LED and a saltwater
powered clock. To be successful, they will need to follow the directions and models provided
and will likely need to troubleshoot any errors in their assembly - possible through multiple
phases of testing, revising and retesting.
--------------------------------------------------------------------------------------------------------------------------------------
Career Connections
Electrochemist
4
Electrochemists develop batteries and electrochemical models to provide electrochemical, thermal,
mechanical, designs and directions for industrial plants, power plants, and even electric vehicle
programs. Electrochemists usually need a masters or bachelors degree and an understanding of
various modeling tools.
Engineer
Engineers are problem solvers who design and create products, buildings, machines, instruments,
and more, for human use and benefits. During the creation of these things, all engineers go through
the process of design, modeling, prototyping, and multiple iterations of modifying their designs until
there is an optimal product or solution. Oftentimes, engineers need to work in teams made up of
people with different subject matter expertise. For example, Electrical Engineers are experts on
electricity generation while electrochemical engineers are experts on designing and running industrial
plants which include an electrolytic stage for the production of chemicals or for power generation.
During the laboratory activities herein, students will have the opportunity to practice teamwork and
their communication skills. The average salary for engineers in Hawaiʻi is $84,631/year.
Electricians
Electricians are trades people who specialize in electrical wiring of buildings, machines, transmission
lines, and related electrical equipment. Electricians may also specialize in wiring ships, airplanes and
other mobile platforms. Typically electricians are employed in the installation of new electrical
components or the maintenance and repair of existing infrastructure. The average salary of an
electrician in Hawaiʻi, as of February 2022, is $33.89/hour.
Green Jobs
Green jobs are jobs that people do to benefit the environment, or to conserve natural resources. Solar
installers, solar technicians, marketers for solar companies, manufacturers that produce renewable
energy equipment, project managers that oversee renewable energy operations, artists that promote
going green, some chemical engineers, some data analysts, and environmental scientists are all
examples of green jobs. Check out the outlook for green jobs here: Where the Green Jobs Grow |
U.S. Department of Labor Blog (dol.gov)
--------------------------------------------------------------------------------------------------------------------------------------
Introduction
Aloha e Energy Explorers!
Welcome to this adventure into the world of hydroelectricity, chemistry, electrochemistry, batteries
and circuits. Throughout these lessons and hands-on laboratory activities, you will discover the
answer to questions like these*:
● What is energy?
● What is electricity?
● How can we use the motion of water to generate electricity?
5

● How can the ocean be used to generate electricity?
● What is the impact of mining on the environment?
● What role do scientists and engineers play in our renewable energy future?
● How can I reduce my energy consumption?
*Take a minute to pose these questions to your students and let them share some of their ideas.
There are no right or wrong answers at this point.
And more importantly, you’ll learn how to ask and investigate the answers to your own
questions to develop solutions.
So what is energy? Well, energy is all around us! It is the power that brings things to life. Energy
describes the work that can be done from energy suppliers like fossil fuels such as oil and gas
(non-renewable), biofuels, nuclear fuels, wind, and solar radiation (renewable), batteries and
hydropower. Electricity is a carrier of energy that is generated by one of the aforementioned
resources. We use electricity to carry energy to power our homes, cities, communities, and everyday
electronics.
In Hawaiʻi, we established the Hawaiʻi Clean Energy Initiative in 2008 (HCEI) with an ambitious goal
to produce 100% of our energy needs from renewable energy sources like solar, wind, geothermal,
hydroelectricity, biomass, and biofuels by the year 2045. As of 2020, 30% of electricity generated in
our state has come from renewable sources and this percentage is growing.
In the labs to follow, you will have an opportunity to test how electricity relates to chemistry, how
circuits control the flow and transportation of electricity from a point of generation, to various targets
that you could find in a home such as lights, motion sensor activated alarms, fans, and doorbells, and
how the ocean around us can be used as a renewable energy resource.
Wait! The ocean? Did you know that the ocean can be used to power our homes, communities,
and cities? How do you think the ocean could be used as a source to generate electrical
energy and why do you think this?
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
*Answers will vary. Some of your students may know about hydroelectricity through water wheel
experiments they’ve done or encountered in the past. They may have seen videos, or read articles
related to this topic. They are less likely to know much about Ocean Thermal Energy Conversion
(OTEC), or electrochemical energy generation from seawater (the topic our the labs to follow)
We’ll come back to this topic of ocean-sourced electrical energy later. But first, let’s think a little bit
more about electricity.
6
Activity: Electricity Exploration (1-class period)
It is 6:00AM and your alarm clock beeps. It is hot and muggy outside, but you can hear the hum of the
air conditioner or a fan keeping you cool. You jump out of bed, and switch on the lights. In just the first
few seconds of being awake, you’ve already used electricity to wake you up, keep you cool, and to
provide light so that you can see while it is still dark. Electricity is everywhere! Whether you live in a
skyscraper in urban Honolulu or a home in the countryside of Molokai, our homes, schools, and
communities are powered by electricity.
To understand electricity, you have to understand the smallest bit of any material called an atom.
Atoms are made up of tiny particles called protons and
neutrons bundled together at the center, and electrons whizzing
around in a “cloud”. Each electron (-) and proton (+) has an
itty-bitty electric charge. If two particles have opposite charges
they will attract, and if they have the same charge, they will repel
each other away. Electrons are special in that they can break free
from their original atom, and hop from one atom to another.
These adventurous electrons are what make all electronics
come to life.
Take a minute to walk around your classroom and if you have
time, your school campus. Identify items that need electricity to work, and where you think (your best
guess) the electricity comes from. Then do some research to determine where the electrical energy
actually comes from. Fill in the data table below:
Data Table 1: Electricity Exploration
Item
Where does the electricity
come from (guess)?
What is the energy source
(research)?
1
Lights
2
Air Conditioner
3
4
5
6
7
8
9
10
7

Tip: You may want to check out the HIDOE Dashboard. Mana - HDOE Dashboard - Ahuimanu
Elementary (manamonitoring.com) to see if your school gets some or all of its energy from
renewable energy sources
Post Activity Assessment:
After your research, did you discover anything that surprised you? ___________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
Answers will vary. If your school is powered by solar, your students may be surprised to learn this.
Key Concepts: Some electrical items like computers, flashlights, and small electronics are powered
by batteries, but most of the electricity we use in our world is generated by large generators driven
by high pressure steam or water pressure (created by burning fossil fuels), or increasingly by wind,
solar, and hydro power. Wires (made of conductive materials) are used to transport this energy to
lights, electrical outlets, air conditioners, and appliances like refrigerators, ovens, and washing
machines. Electricity may be transported over very large distances such as a power station miles
away, to very tiny distances, like through circuits in your computer. While we cannot see electricity,
that does not mean it is magic - it’s science, and it’s really easy to learn.
In the labs that follow, we’ll investigate how hydropower works, build our very own battery, and
engineer a saltwater powered clock. Let’s jump into hydropower next.
Scientific Research: Hydroelectricity (1-class period)
Scientists conduct experiments and make observations in order to discover the answers to
questions that people generally do not know the answers to, or to broaden our understanding of a
phenomena that we have a limited understanding of. What is something that you always wanted
to know more about? _____________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
8

Before jumping into experiments and/or field research, which can be very expensive, scientists must
start by learning all they can about what is already known about their topic of interest. This process is
called the literature review. In a literature review, scientists will learn everything they can about a
topic by reading textbooks, watching videos, occasionally through news articles, and primarily by
reading peer-reviewed publications (primary literature). After reading hundreds if not thousands of
texts, scientists will have to synthesize and summarize their findings and use that information to
present to others why there is a need for their proposed research or study.
In this activity, you will practice reviewing online, text, and video resources to learn about
hydroelectricity.
Here are some guiding questions to help you begin your research:
1. What is hydroelectricity or hydroelectric power?
2. Why is hydropower considered a renewable resource?
3. Does Hawaii have any hydroelectric plants?
4. How can the ocean be used to generate electricity?
5. How is hydroelectric power similar to wind power? How is it different?
Hydroelectricity Research (Literature Review)
Information
Source
(Citation)
Is your source
credible? How
do you know?
What did you learn?
Could this be a way to
generate electricity in
Hawaii? Why or why
not? What would the
challenges be?
1
2
3
4
5
Summary of findings
9
After your research, did you discover anything that surprised you?
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
Answers will vary.
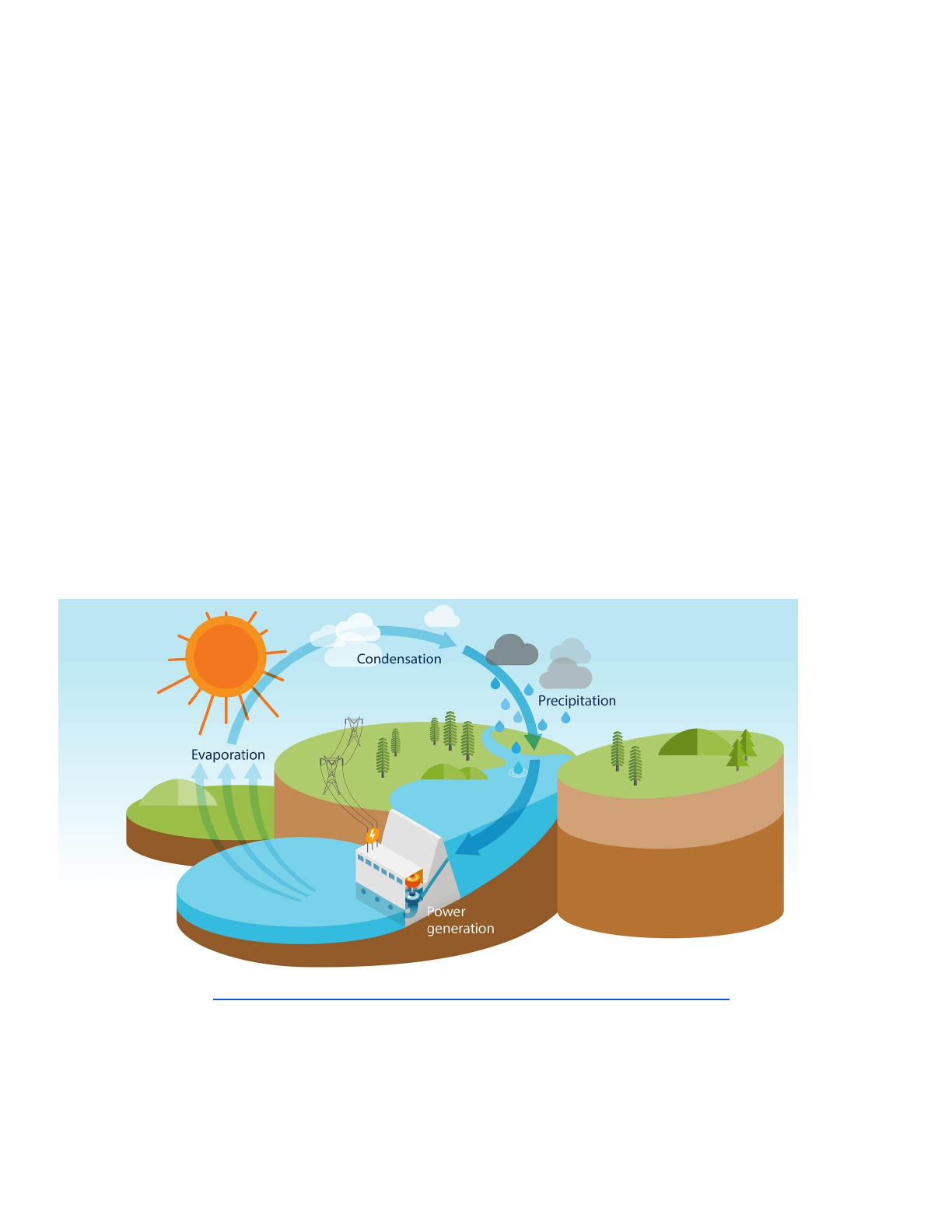
Key Concepts: Hydropower is the production of electrical energy through the use of gravitational
force of falling or flowing water (kinetic energy). Water is about 800 times denser than air, so even a
slow flowing stream, or moderate tides, can produce a considerable amount of energy by spinning a
turbine (like a windmill). That mechanical energy is transferred to an electricity generator and the
electrical energy is transported from the powerstation through power lines to power your home.
Hydrologic Cycle - Hydropower | Advantages | Hydro-Québec (hydroquebec.com)
10
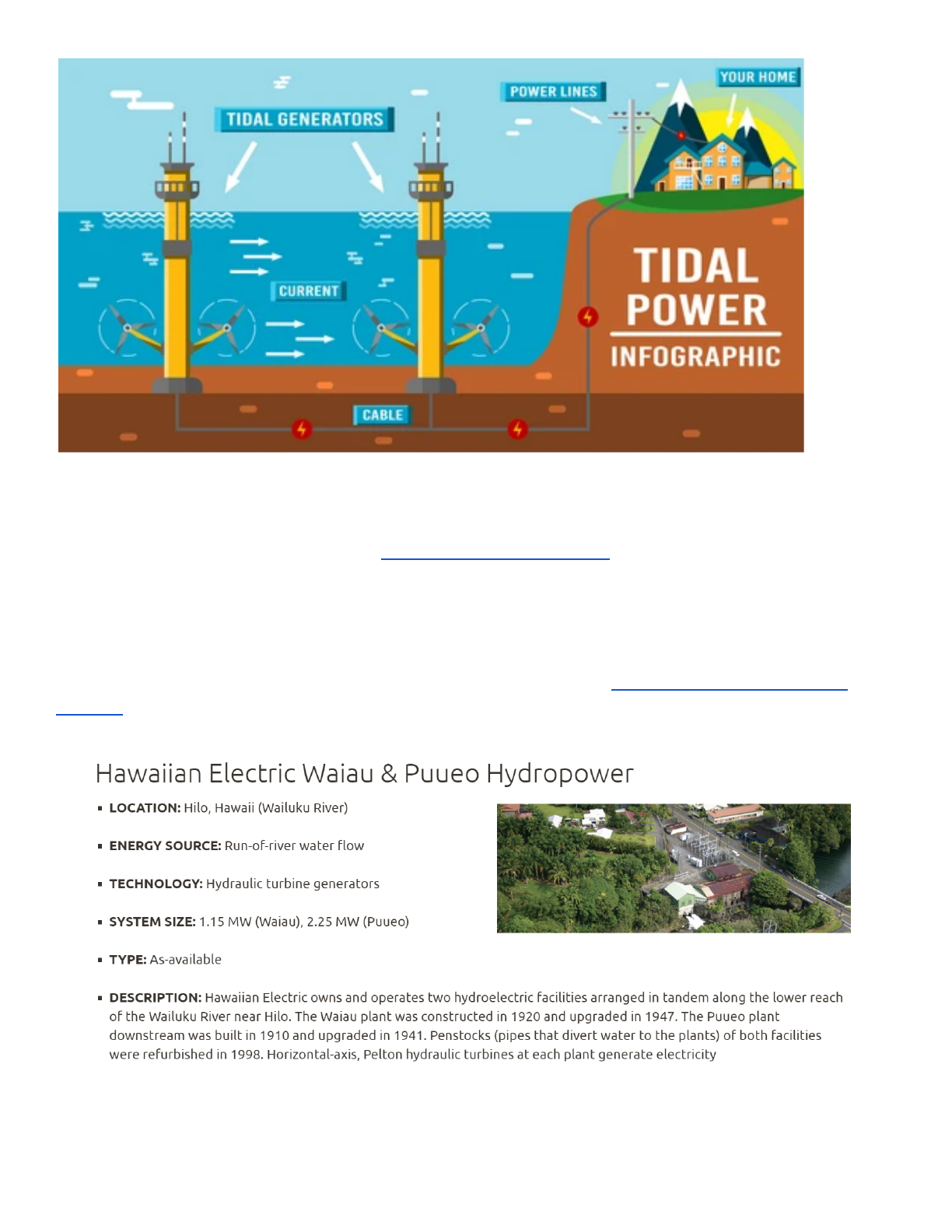
Tidal Power Infographic (Free shutterstock graphic). Tides move a massive amount of water each
day, but at low speeds which make energy conversion difficult. Tides are very predictable though
which gives it a big advantage over other types of renewable energy sources like wind and solar.
Extra Resource: Check out this video: How Hydroelectricity Works.
Renewable Energy Connection: Moving water is a natural part of the hydroelectric cycle, and it is a
renewable source of mechanical energy that can be harnessed and transformed into electrical
energy. Hydroelectric power is a small, but growing source of renewable energy. It makes up about
1.4% of Hawaii’s renewable energy portfolio as of 2022. Check out Hydroelectricity | Hawaiian
Electric for more examples like this one below:
Our state has the potential to increase our percentage of hydroelectric energy generation if we can
harness mechanical energy from the ocean’s waves and tides. In Hawaii, we have highly consistent
11

wave energy resources, and currently wave energy generation is in the experimental phase.
Hawaiian Electric partnered with the Office of Naval Research to test wave energy generation off of
Kaneohe Marine Corps Base of Oahu. This experimental setup employs the bobbing motion of buoys
to drive an electrical generator. Ocean Energy | Hawaiian Electric
Another way the ocean can be
used to generate electricity is
through Ocean Thermal Energy
Conversion (OTEC) which uses the
temperature difference between
cold deep seawater and warm
coastal waters to produce
electricity. Currently OTEC systems
are extremely expensive and there
are no commercial scale OTEC
plants anywhere in the world.
However, Hawaii has experimented
with OTEC since the 1970s at the
Natural Energy Laboratory of
Hawaii (NELHA) on Hawaii Island.
It was found to be technically feasible but prohibitively expensive in comparison to oil prices at that
time. Maybe there will be a technological breakthrough in the future that can bring costs down.
Ocean Energy | Hawaiian Electric Want to learn more about harnessing the power of the oceans?
Check out this book, The Next Wave.
Now that you’ve learned about
hydropower, did you know that there is
ANOTHER way that we can use the ocean
to produce electricity that doesn’t involve
mechanical or thermal energy? In the next
four, hands-on laboratories, you’re going
to run several chemistry experiments to
discover how we can use chemical energy
to produce electricity. At the end of these
activities, you will build your very own
saltwater powered clock!
For these labs, we’ll be using the
Electrochemistry Lab Kit by KiwiCo. Let’s
take a look at what all is included in this
kit. This kit provides a fun, tangible way for
you to research how chemical energy and electricity are related by doing your experiments.
Electrochemistry Kit Discovery (1-class period)
12
NGSS Performance Expectations
MS-ESS3-3 Apply scientific principles to design a method for monitoring and minimizing a human impact on the
environment.
Students are asked to research the metals and chemicals in this kit to determine where they come from, and are
challenged to think about the environmental impact of mining these materials as well as potential solutions.
This exercise focuses on the following three dimensional learning aspects of NGSS
Science & Engineering Practices
Disciplinary Core Idea
Crosscutting Concepts
Constructing Explanations and Designing
Solutions
Constructing explanations and designing
solutions in 6–8 builds on K–5 experiences
and progresses to include constructing
explanations and designing solutions
supported by multiple sources of evidence
consistent with scientific ideas, principles,
and theories.
● Apply scientific principles to
design an object, tool, process
or system.
ESS3.C: Human Impacts on Earth
Systems
● Human activities have
significantly altered the
biosphere, sometimes
damaging or destroying natural
habitats and causing the
extinction of other species. But
changes to Earth’s
environments can have
different impacts (negative and
positive) for different living
things.
● Typically as human populations
and per-capita consumption of
natural resources increase, so
do the negative impacts on
Earth unless the activities and
technologies involved are
engineered otherwise.
●
Cause and Effect
● Relationships can be classified
as causal or correlational, and
correlation does not
necessarily imply causation.
Connections to Engineering,
Technology, and
Applications of Science
Influence of Science, Engineering, and
Technology on Society and the Natural
World
● The uses of technologies and
any limitations on their use are
driven by individual or societal
needs, desires, and values; by
the findings of scientific
research; and by differences in
such factors as climate, natural
resources, and economic
conditions. Thus technology
use varies from region to
region and over time.
HĀ outcomes practiced: 1. Belonging - students will work in teams, actively participate, and practice communicating with clarity and
confidence. 2. Responsibility - students will be active learners, encouraged to ask for help, will need to set goals and complete tasks with
their team. 4. Aloha - students should learn to appreciate the gifts and talents of their team members, make everyone feel welcomed and
heard, be respectful of all ideas, and share responsibilities.
Before the Activity
● Have students select their teams of four
● Make sure that each team member has a chance to participate in the hands-on portion of the
lab.
Materials List - For this activity, each group will need:
● One KiwiCo Electrochemistry Kit (included)
Procedure: Identify Parts
1. Open up the electrochemistry kit and remove all the pieces from the box
2. Match the items from the kit to the parts list on Page 2 and 3 of the manual
13
3. Are there any parts that are unfamiliar to you (you don’t know what they are, what they do, or
how they work)? If so, check the box next to those parts in column 1 of the table below.
4. For all the listed items, you may want to do some research to learn more about what they are,
where they come from, and what they can be used for.
Electrochemistry Kit Exploration
Part
Research - What is it? Where does it come from? What can it
be used for? If it is a chemical, you may also want to look up the
chemical formula
Voltmeter
Alligator Clip
LED
Wires
Switch
LCD Clock
Cups, Vials, Beakers,
Scoop
Steel Wool
Aluminum Foil
Magnesium
Copper
Zinc
Carbon
14
Copper Sulfate
Sodium Chloride
In Labs 1-4 you will be doing real chemistry experiments. All of the items in your kit are safe to
use, however:
● Be sure to keep chemicals out of your eyes and rinse immediately with water (remove contacts
if you wear them). Place your entire face in water and flush your eyes. (You may want to wear
safety goggles if you have them)
● Do not taste or try to eat any of the chemicals in this kit.
● Do not use any outside electrical components or chemicals for these experiments. Only use
what is provided in this kit.
Kit Discovery Renewable Energy Connection: Most of the items in this kit came from materials
that had to be mined or extracted from the earth. In reality, the technical components and industrial
supplies for renewable energy systems also have to be mined from the earth whether it be the metals
for turbines and generators, silicon and other materials for solar cells, lithium and other materials for
batteries. Everything that we build will have some environmental impact. Can you think of some
ways that we could reduce our environmental impacts from mining raw materials? _________
________________________________________________________________________________
________________________________________________________________________________
This kit and following experiments also introduced you to constraints. You will only be permitted to
use the supplies that have been provided to you. In reality, all engineers are limited by time and
resources. Engineers have to work with what they have in the time they have available to them and
this includes chemical engineers. Constraints often inspire innovation and creativity. Alright, are you
ready to do some chemistry? In Lab 1, you’re going to use written instructions, diagrams, and models
to learn more about chemical changes and reactions through experimentation and observations.
Lab 1: Aluminum Eater - Chemical Reactions (1-class period)
NGSS Performance Expectations
MS-PS1-5 Develop and use a model to describe how the total number of atoms does not change in a chemical reaction
and thus mass is conserved. [Emphasis is on the law of conservation of matter and on drawings, including physical
forms, that represent atoms - does not include use of atomic masses, balancing equations, or intermolecular forces.]
Students are asked to conduct a simple, but dramatic, chemical reaction that will eat up aluminum foil. They will
witness a redox (oxidation-reduction) reaction, and be asked to draw a representation of what happened to all of the
atoms in this experiment.
MS-PS1-2 Analyze and interpret data on the properties of substances before and after the substances interact to
determine if a chemical reaction has occurred. [Assessment is limited to analysis of the following properties: density,
15
melting point, boiling point, solubility, odor, flammability].
Students will need to record their observations of changes in properties of a substance that confirms that a chemical
reaction has taken place. In this case, they’ll be looking for changes in properties in the aluminum foil.
This exercise focuses on the following three dimensional learning aspects of NGSS
Science & Engineering Practices
Disciplinary Core Idea
Crosscutting Concepts
Developing and Using Models
Modeling in 6–8 builds on K–5 and
progresses to developing, using and revising
models to describe, test, and predict more
abstract phenomena and design systems.
● Develop a model to describe
unobservable mechanisms.
Analyzing and Interpreting Data
Analyzing data in 6–8 builds on K–5 and
progresses to extending quantitative
analysis to investigations, distinguishing
between correlation and causation, and
basic statistical techniques of data and error
analysis.
● Analyze and interpret data to
determine similarities and
differences in findings.
Connections to Nature of Science
Science Models, Laws, Mechanisms, and
Theories Explain Natural Phenomena -
Laws are regularities or mathematical
descriptions of natural phenomena.
Scientific Knowledge is Based on
Empirical Evidence - Science knowledge is
based upon logical and conceptual
connections between
PS1.B: Chemical Reactions
● Substances react chemically in
characteristic ways. In a
chemical process, the atoms
that make up the original
substances are regrouped into
different molecules, and these
new substances have different
properties from those of the
reactants.
● The total number of each type
of atom is conserved, and thus
the mass does not change.
PS1.A: Structure and Properties of Matter
● Each pure substance has
characteristic physical and
chemical properties (for any
bulk quantity under given
conditions) that can be used to
identify it.
Energy and Matter
● Matter is conserved because
atoms are conserved in
physical and chemical
processes.
Patterns
● Macroscopic patterns are
related to the nature of
microscopic and atomic-level
structure.
HĀ outcomes practiced: 1. Belonging - students will work in teams, actively participate, and practice communicating with clarity and
confidence. 2. Responsibility - students will be active learners, encouraged to ask for help, will need to set goals and complete tasks with
their team. 4. Aloha - students should learn to appreciate the gifts and talents of their team members, make everyone feel welcomed and
heard, be respectful of all ideas, and share responsibilities.
Prerequisite Knowledge
● Take a minute to discuss the information on pages 4-5 of the electrochemistry manual. This
manual uses cooking pancakes as an example of chemical changes. You may want your
students to come up with a list of chemical changes they have observed in their everyday life.
● You may want to go over what makes up an atom and that molecules are one or more atoms
that are stuck together like water (H20).
Before the Activity
● Have students select their teams
● Make sure there is enough space for the experiment in the center and for each student to
make observations
16

● Gather the materials listed below (page 6 of the KiwiCo Manual)
Materials List - For this activity, each group will need:
● Mess Matt
● Beaker
● Cup
● Scoop
● 1 Stir Stick
● Copper Sulfate
● Sodium Chloride
● Aluminum Foil
● Hot water (not included)
● Scissors (not included)
Potential Safety Issues
● This is a quick and dramatic chemical reaction. Do not have your eyes too close to the cup.
● This reaction will produce heat, do not touch the cup during the reaction.
● Be careful that you do not knock over the cup with the solution in it.
● Be careful to make enough of a “bowl” shape in the aluminum foil so that the solution neither
overflows or falls through gap between the foil and the cup (there shouldn't be any gaps
between the foil and the plastic cup)
Potential Obstacles
● If the water is too cold, the reaction may not be dramatic. You have enough supplies to run this
experiment twice so you may want to test different water temperatures.
Procedure
1. Layout the mess mat
2. Follow Steps 1-6 in the KiwiCo Manual (pages 6-8).
Embedded Activity Assessment:
1. What color is the copper sulfate and salt solution? _________________ (blue)
Procedure Continued:
3. Follow Steps 7-9 in the KiwiCo Manual (page 9) - make mental and physical notes.
NOTE- THIS REACTION WILL HAPPEN QUICKLY SO BE READY TO MAKE
OBSERVATIONS.
Embedded Activity Assessment Continued:
2. How long did it take for the reaction to start? ____________________ (immediately)
17

3. How long did it take for the reaction to eat through the aluminum foil __________(10
seconds)
4. Describe what you saw as the reaction started ________________________________
______________________________________________________________________
_____________________________________________________________________
______________________________________________________________________
5. What do you see in the beaker when the reaction is done? _______________________
______________________________________________________________________
______________________________________________________________________
______________________________________________________________________
6. What does the aluminum foil that is left look like? ______________________________
______________________________________________________________________
______________________________________________________________________
______________________________________________________________________
Answers will vary but it should look like it has holes in it and black like it has been
burned (oxidized). If you see red, that is rust.
Optional Extension Challenge: Repeat the experiment with cold water instead of hot water. How
does this change the reaction? _______________________________________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
(Slower, less dramatic, if very cold water, might not work well at all.)
Clean up - throw away the aluminum foil and the materials in the beaker. Rinse the beaker, cup, and
stir stick in cold water and keep these items to use again.
Post-Lab Assessment
18
You just observed a chemical change and made aluminum foil disappear! Or did you!? While it may
appear that the aluminum foil disappeared, it is actually still there, just in a different chemical form. A
fundamental fact in chemistry (law) is that we cannot increase or decrease the amount of matter
present in the universe. That means before, during, and after this chemical reaction, the number of
copper atoms and aluminum atoms stayed the same. Draw a picture or model below that illustrates
this conservation of matter.
(Some of the aluminum atoms are now a part of the solution in the beaker and the copper atoms in
the copper sulfate solution formed the reddish-brown materials that you see.)
19
Key Concepts: This reaction was an example of two different chemical reactions called
oxidation-reduction or “redox” for short. Redox reactions are extremely common in nature. In an
oxidation reaction, an atom will lose electrons and the lost electrons have to go somewhere which is
why a reduction reaction always occurs as well. Reduction reactions involve an atom gaining
electrons. In this experiment. The aluminum was in an oxidation state (lost electrons) and the copper
was in a reduction state (gained electrons). Combustion or burning is another example of a redox
reaction. Most redox reactions involve the transfer of oxygen atoms, hydrogen atoms, or electrons.
Fun Fact - Have you ever seen an old book where the pages have turned yellow or even brown? The
paper is actually burning in slow motion via a redox reaction.
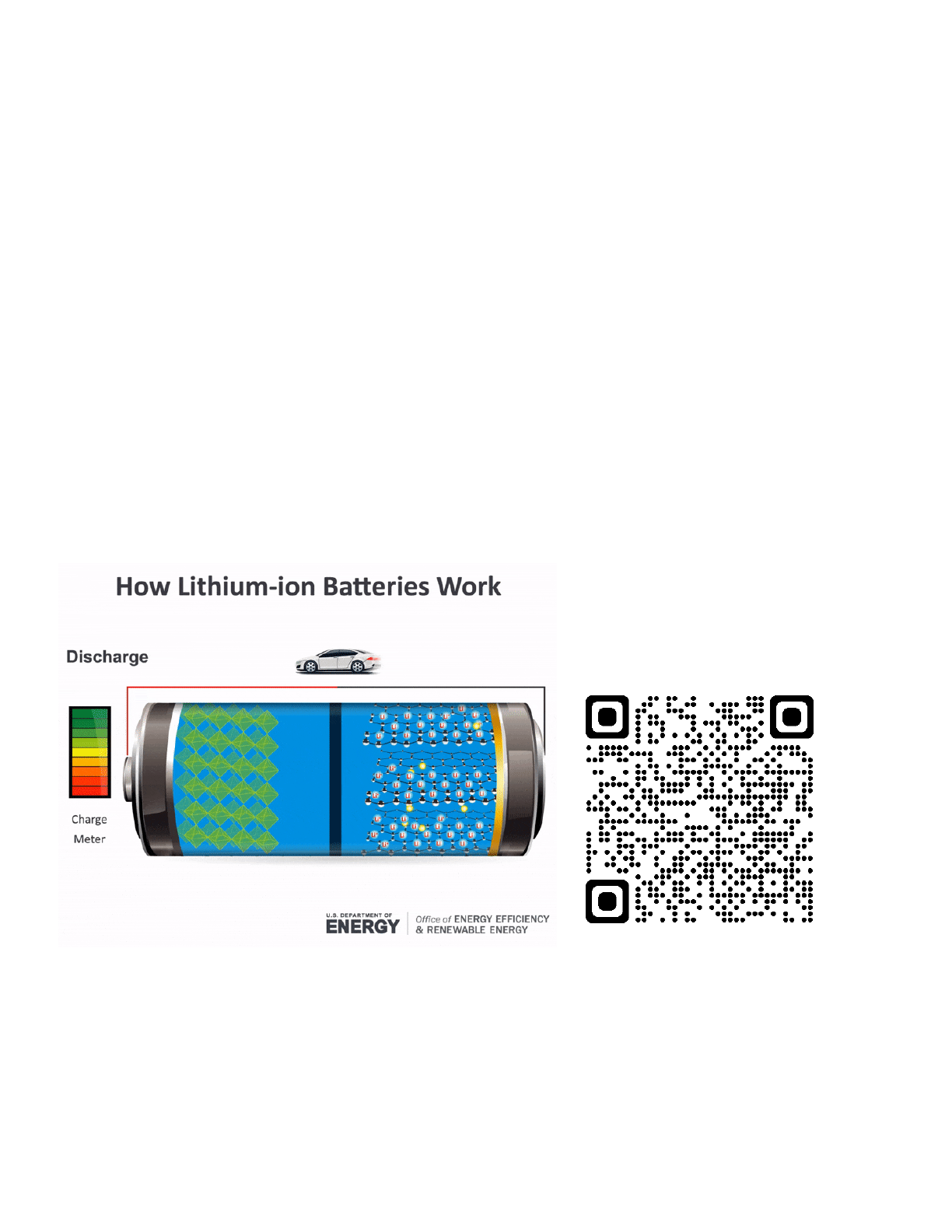
Renewable Energy Connection: Batteries produce electric current from electrochemical oxidation
and reduction reactions. A battery essentially converts chemical energy into electrical energy. If you
take a look at a battery, you will notice that it has a negative node (-) and a positive node (+). At the
negative electrode, an oxidation reaction takes place and electrons flow out of the cell, through a
circuit, and return to the battery at the positive electrode.
Batteries are going to play an enormous role in our renewable energy future from storing electrical,
wind and hydropower energy to powering electric vehicles in the transportation sector. Can the QR
code below to see an animated .gif for the graphic below.
In Labs 3 and 4, you will make your very own batteries! But first, let’s learn how to use your voltmeter
and explore voltage and electric potentials of various conductive materials.
20
Lab 2: Voltage Tests (1 class period)
NGSS Performance Expectations
Voltage The NGSS focuses on a conceptual understanding of core ideas. Voltage, resistance, and current describe
the movement of a charge, and more broadly, the transfer of energy. These words and their respective symbols and
definitions can be introduced and applied for instructional purposes as needed.
Oxidation/Reduction The NGSS do not include specific names of chemical reactions and instead focus on conceptual
understanding of how chemical reactions occur. This ensures that students have a conceptual understanding that they
can apply to any type of chemical reaction. Classes of chemical reactions such as oxidation and reduction, acid and
base, or decomposition and synthesis can be used in instruction depending on the context, but instruction should
ensure that students have an understanding of the underlying concepts.
Related PEs and Bundles: MS-PS1-2 Matter and its Interactions
HĀ outcomes practiced: 1. Belonging - students will work in teams, actively participate, and practice communicating with clarity and
confidence. 2. Responsibility - students will be active learners, encouraged to ask for help, will need to set goals and complete tasks with
their team. 4. Aloha - students should learn to appreciate the gifts and talents of their team members, make everyone feel welcomed and
heard, be respectful of all ideas, and share responsibilities.
Prerequisite Knowledge
● A voltmeter tells you the voltage between two points. You can think of voltage as electrical
pressure - it is what moves electrons through a circuit. See the KiwiCo manual page 21.
● You may want to practice testing the Voltmeter with an AA battery. See page 26 of the KiwiCo
manual for a few suggestions.
Before the Activity
● Have students select their teams
● Make sure there is enough space for the experiment in the center, and for each student to
make observations. Every student should participate in taking measurements.
● Gather the materials listed below (page 16 of the KiwiCo manual)
Materials List - For this activity, each group will need:
● Mess Matt
● Beaker
● Clips
● Voltmeter
● Carbon strip
● Zinc strip
● Aluminum foil
● Steel wool
● Sodium chloride (salt)
● warm water (not included)
● Permanent Marker (optional - not included)
21

● AA battery (optional - not included)
Potential Safety Issues
● None. This is a very safe experiment.
Potential Obstacles
● The alligator clips must be fully in contact with the metal strip being tested.
● Make sure metal strips are partially submerged in the water/solution.
● Make sure the metal strips are not touching each other.
Procedure
1. Lay out the mess mat
2. Follow Steps 1 - 5 in the KiwiCo manual (pages 16-18)
Embedded Activity Assessment
1. What voltage did you measure?
Magnesium-carbon in plain water ______________________________
Voltage (V) = Current (I) x Resistance (R). Volts are a measure of potential energy. The higher the
volts, the more potential energy is stored.
3. Follow Steps 6 in the KiwiCo Manual (page 19)
2. What was the highest voltage you read for Magnesium-carbon in salt water? _______
3. What do you think will happen when you swap the wires? Make a prediction: ________
______________________________________________________________________
______________________________________________________________________
4. Follow Steps 7-8 in the KiwiCo Manual (page 20). Was your prediction correct? ____________
5. Follow Steps 9 - 14 in the KiwiCo Manual (pages 22-25)
4. What voltage did you see for copper-zinc in salt water ___________________ (~0.4)
5. How does the copper-zinc voltage compare to the magnesium-carbon voltage?
__________________________________ (less)
22
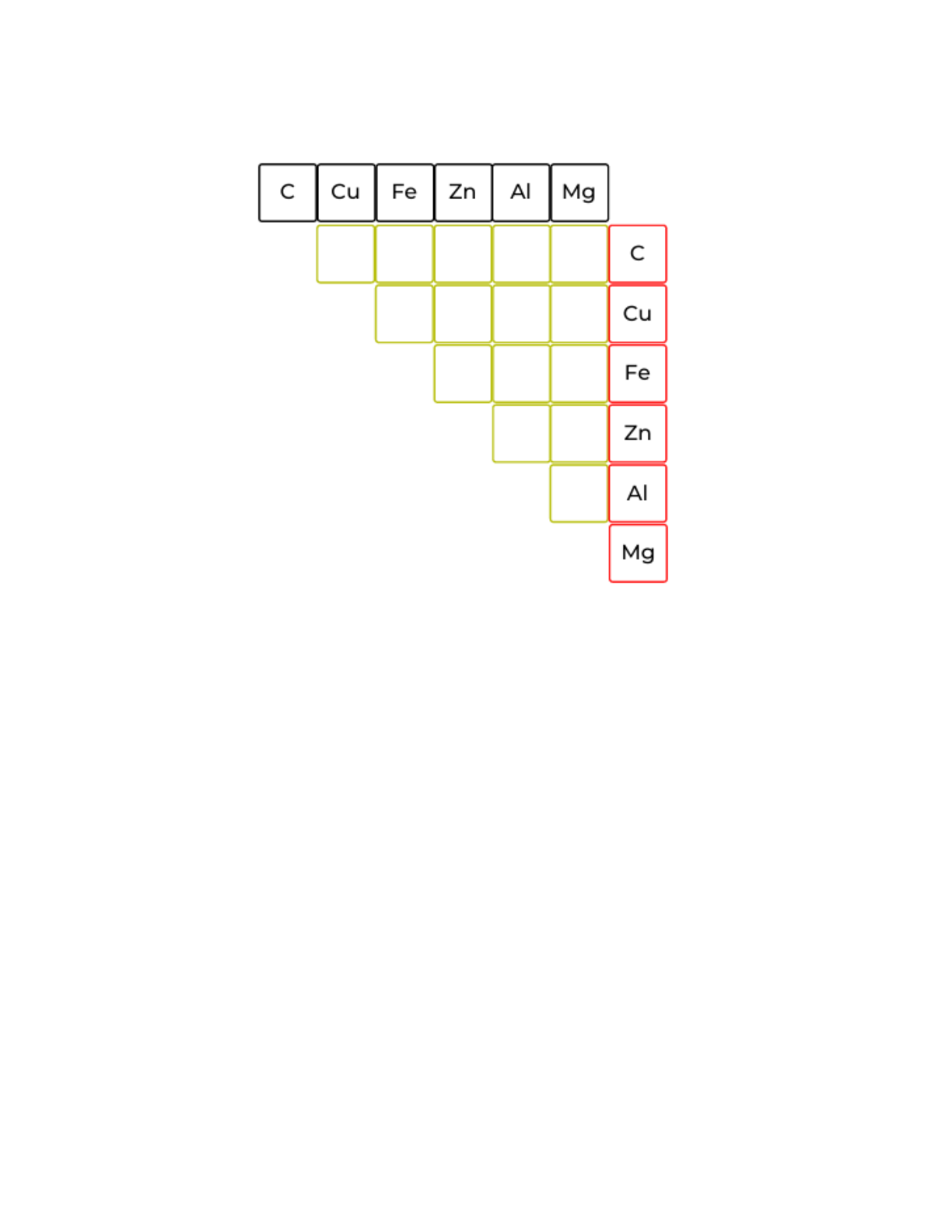
6. Fill out the following table with the voltages you measure. Remember, if you get a zero
reading, swap the wires, and make sure the clips are in good contact with the metal
strips. (Note: Fe is the iron in your steel wool)
7. Which combination gives the highest voltage reading? __________________________
(Mg-C)
8. Which combination gives the lowest voltage reading? __________________________
(Cu-C)
9. Which combination of metals would you use to power a clock and why? ____________
______________________________________________________________________
______________________________________________________________________
______________________________________________________________________
Hint - Remember that the higher the volts, the more potential energy is stored.
Optional Extension - Test other materials in your classroom such as coins, pencil lead, jewelry,
anything that you don’t mind putting in saltwater.
23

Key Concepts: In this experiment you created a closed circuit using metal strips, wires, and a
conductive solution. A circuit is like a racetrack for electrons. If there is a break in the circuit at any
point, then electrons cannot flow through the track. That’s why if your alligator clips were not in good
contact with the metal strip, or the metal strip was not in the saltwater solution, you would get a 0
voltage reading.
If you can break an electron free from an atom and force it to move, you can create electricity.
The voltmeter is a tool that allows you to measure the voltage of the electrical current running through
the circuit. Elements that are conductors such as copper, silver, and gold, have very mobile
electrons. We use these materials to create wires to help electrons flow. Electrons in elements with
low conductivity (insulators) do not break free very easily, preventing the flow of electrons. These are
materials like glass, rubber, plastic, and air. In this experiment electrons flow from the elements with
higher electric potential to elements with lower electric potential.
Also in this experiment, the conductive solution that was used was saltwater. When the
sodium-chloride was mixed into the water, it broke down into sodium ions and chloride ions. An ion is
just an atom or molecule that loses or gains electrons. Ions are fantastic at moving electrons
around. This is why the saltwater solution filled with ions allows electricity to move through the circuit
efficiently. Look back at your pure water vs saltwater voltage measurement. The voltmeter readings
tell you that there are electrochemical reactions happening. Just like you observed in Lab 1:
Aluminum eater, these electrochemical reactions in this experiment are also redox reactions that are
moving electrons around.
Renewable Energy Connection: The greater the difference in electric potential between the two
metals, the more energy you can get from those combinations of elements. This is important
fundamental knowledge to have if you want to use redox reactions to create a battery. Also, just like
in this experiment, the salt ions in seawater can be used to move electrons around to generate an
electric current. There is a startup company in Hawaii, Polu Energy, that is testing the possibility of
using seawater and groundwater to produce renewable energy for small-scale energy needs.
24

Polu Energy Video Link
Next, in Labs 3 and 4, we’re going to build on these voltage experiments to build a coin cell battery
that will power a LED flashlight and a saltwater solution powered clock! Let’s dive in!
Lab 3: Coin Cell Battery Flashlight
NGSS Performance Expectations
MS-ETS1-1 Define the criteria and constraints of a design problem with sufficient precision to ensure a successful
solution, taking into account relevant scientific principles and potential impacts on people and the natural environment
that may limit possible solutions.
Students will build a coin cell (voltaic pile) powered flashlight aka an electrochemical battery that will power a LED.
Their constraints will be the materials provided in the kit. They may need to troubleshoot their designs and make
changes until the LED lights up properly.
This exercise focuses on the following three dimensional learning aspects of NGSS
Science & Engineering Practices
Disciplinary Core Idea
Crosscutting Concepts
Asking Questions and Defining Problems
Asking questions and defining problems in
grades 6–8 builds on grades K–5
experiences and progresses to specifying
relationships between variables, and
clarifying arguments and models.
● Define a design problem that
can be solved through the
development of an object, tool,
process or system and
includes multiple criteria and
constraints, including scientific
knowledge that may limit
possible solutions.
ETS1.A: Defining and Delimiting
Engineering Problems
● The more precisely a design
task’s criteria and constraints
can be defined, the more likely
it is that the designed solution
will be successful.
Specification of constraints
includes consideration of
scientific principles and other
relevant knowledge that are
likely to limit possible solutions.
Energy and Matter
Energy can be transferred in various ways
and between objects.
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Systems and System Models
Models can be used to represent systems
and their interactions – such as inputs,
processes, and outputs – and energy and
matter flows within systems.
Influence of Science, Engineering, and
Technology on Society and the Natural
World
All human activity draws on natural
resources and has both short and long-term
consequences, positive as well as negative,
for the health of people and the natural
environment.
25
The uses of technologies and limitations on
their use are driven by individual or societal
needs, desires, and values; by the findings
of scientific research; and by differences in
such factors as climate, natural resources,
and economic conditions.
HĀ outcomes practiced: 1. Belonging - students will work in teams, actively participate, and practice communicating with clarity and
confidence. 2. Responsibility - students will be active learners, encouraged to ask for help, will need to set goals and complete tasks with
their team. 4. Aloha - students should learn to appreciate the gifts and talents of their team members, make everyone feel welcomed and
heard, be respectful of all ideas, and share responsibilities.
Prerequisite Knowledge
● You may want to do a quick refresher on what electricity is. Here is an excellent tutorial with
some simple animations: What is Electricity? - learn.sparkfun.com
Before the Activity
● Break up into teams
● Gather the following materials:
Materials List - For this activity, each group will need (KiwiCo Manual page 28):
● Mess Mat
● Beaker
● Scoop
● Stir Stick
● Chipboard Disks
● Sandpaper
● Black Sticky Foam
● Gray Sticky Foam
● Black Double-Stick Foam
● Plastic Tube
● Voltmeter
● Connector Wires
● Switch
● LED
● Sodium Chloride (salt)
● Copper Disks
● Zinc Disks
● Warm Water (not included)
● Scissors (not included)
Potential Safety Issues
● None
26

Potential Obstacles
● Sanding the copper and zinc discs will take some time. Students will need to be patient and
will need to make sure the shiny protective coating is heavily scratched up. If they get tired,
they should take turns.
● Do not mix the two discs up. Zinc discs are gray and the copper discs are orange.
Procedure
1. As before, start by laying out the mess mat.
2. Follow Steps 1-9 in the KiwiCo Manual (pages 28-31). Pay special attention to tips in red font.
Embedded Activity Assessment
Did you get a voltage reading of 0.3 to 0.6 volts? ____________________ If not, make sure the red
alligator clip is touching the copper disc and the black one is touching the zinc.
3. Repeat Steps 4-9 to make a total of five cells.
4. Check each cell with the voltmeter. If any cell does not produce a voltage, you’ll need to
troubleshoot. Troubleshooting is an important part of the engineering process. See the
troubleshooting tips on page 32 of the KiwiCo manual.
5. Follows Step 11 (KiwiCo manual page 32). Make sure that all the cells are facing in the same
direction so that the copper and zinc are always back to back.
6. Test the voltage of your voltaic pile. It should be about 1.0V (if higher, that’s great!)
You just built a 1.0V battery! Way to go engineers! Now we need to wire this battery up in a circuit to
power the LED. This time, our circuit will have an on-off button that will break the circuit to turn the
light off, or complete the circuit to turn the light on.
7. Follow Steps 12 - 30 in the KiwiCo manual (page 33 - 38). Pay careful attention to text in the
red font.
Post-Activity Assessment
1. Did your coin battery powered flashlight work the first time? _________ If not, what steps did
you take to troubleshoot the problem? What troubleshooting action(s) worked to solve the
problem?____________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
(answer will vary)
2. All Electrical components require a certain amount of voltage to work properly. In this test, you
saw that your LED needed at least 1.0V to light up. Over time, your LED’s beam of light will
weaken. Why do you think this is? _______________________________________________
27
__________________________________________________________________________
__________________________________________________________________________
(As the cells dry out, there will not be any ions in solution to help move the electrons along.)
Key Concepts: This activity builds upon the basic electrochemistry concepts that were learned in
Labs 1 and 2 and added in an introduction to the engineering design process. The constraints in this
exercise were the materials that were available in the kit. Engineers always have constraints in terms
of materials, and will often have to troubleshoot an assembly, sometimes through multiple iterations of
troubleshooting, in order to get their design to perform as needed. Sometimes when something does
not work the first time, students are tempted to give up because it “doesn’t work”. With just a few
modifications, it is often possible to develop a solution to solve a problem. This takes practice,
patience, and persistence.
Career Connection: In this activity, you were thinking like
electrical engineers! Electrical engineers design circuits for
not only electrical components in your home, but also for
robots, electric cars, computers, cell phones, prosthetics,
space shuttles, drones, video game consoles, and more.
Renewable Energy Connection: In this engineering
experiment, you recreated a version of the very first battery,
known as a voltaic pile, that can supply a continuous electric
current to a circuit. It was invented by an Italian doctor and
scientist in 1780. You can read about Luigi Galvani and how
he came up with his invention on pages 40 - 41 of the KiwiCo
manual.
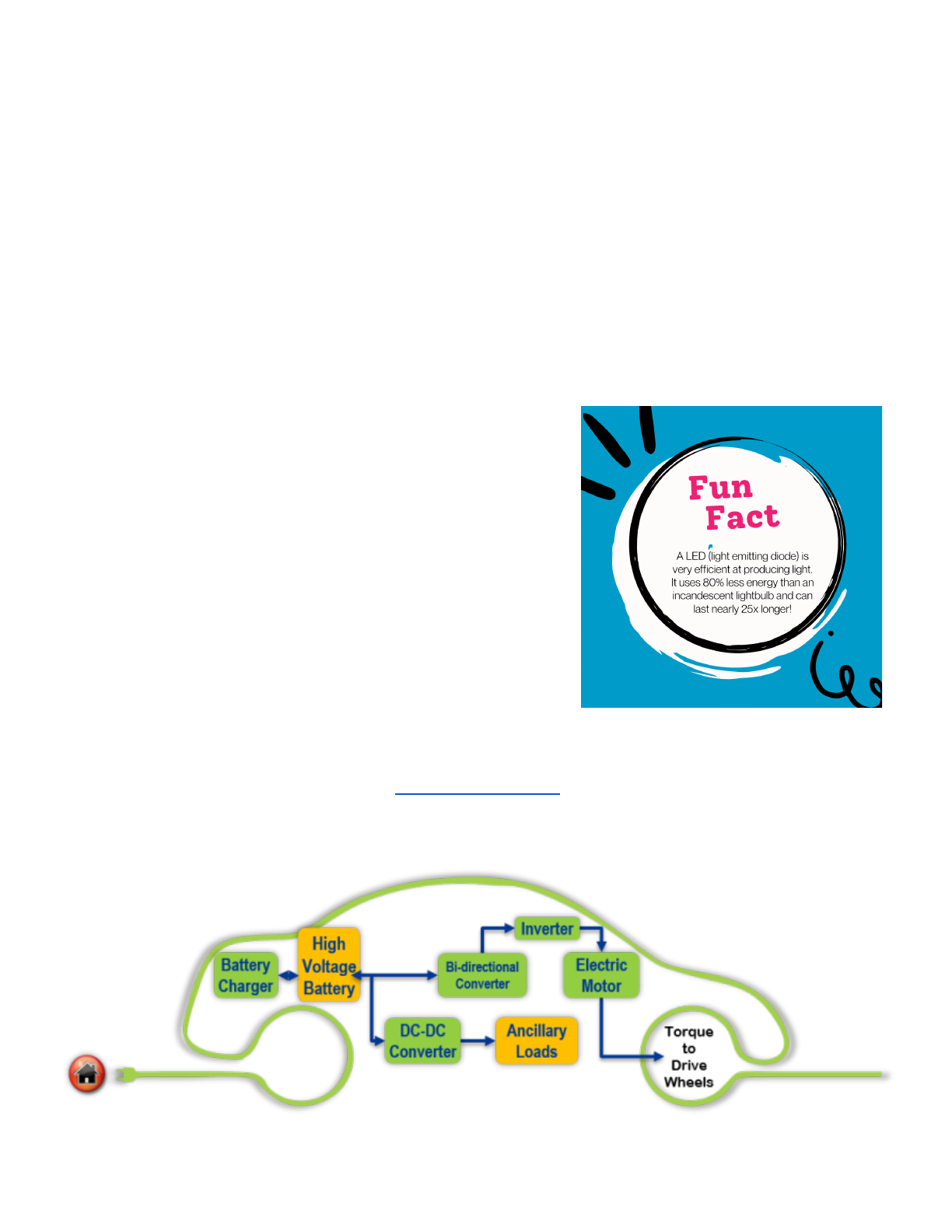
Batteries are likely to play a big role in our renewable energy future. The Hawaiʻi State Energy Office
has taken a leadership role in advancing clean transportation including facilitating the deployment of
zero emission, electric vehicles and charging infrastructure. Using electric vehicles, that are powered
by rechargeable batteries, will help reduce fossil fuel consumption in the transportation sector.
28
Graphic Source
In terms of powering our homes, schools, and other infrastructure with renewable energy, the sun is
not always shining to produce solar power, and the wind is not always blowing to produce wind
power. Batteries that can store energy produced by these renewable sources will be a key component
of a 100% renewable energy future. Innovations in battery technologies are desperately needed. Will
you be one of the engineers who design better batteries?
Optional Deeper Dive: What are batteries made of? (MS-PS1-3) Have students gather and make
sense of information to describe that synthetic materials used to make batteries come from natural
resources and impact society.
Extra Resource: How batteries work - Adam Jacobson - YouTube
Alright Electrochemists. Are you ready for the grand, final, challenge? Let’s put everything that we
have learned together to build a saltwater powered clock!
Lab 4: Saltwater Powered Clock (1 class period)
NGSS Performance Expectations
MS-ETS1-1 Define the criteria and constraints of a design problem with sufficient precision to ensure a successful
solution, taking into account relevant scientific principles and potential impacts on people and the natural environment
that may limit possible solutions.
Students will build a saltwater power clock with constraints being the supplies they have on hand.
MS-PS1-5 Develop and use a model to describe how the total number of atoms does not change in a chemical reaction
and thus mass is conserved. [Emphasis is on the law of conservation of matter and on drawings, including physical
forms, that represent atoms - does not include use of atomic masses, balancing equations, or intermolecular forces.]
Students are asked to draw a model of their clock showing what is going on on a molecular level in terms of electrons.
This exercise focuses on the following three dimensional learning aspects of NGSS
Science & Engineering Practices
Disciplinary Core Idea
Crosscutting Concepts
Developing and Using Models
Modeling in 6–8 builds on K–5 and
progresses to developing, using and revising
models to describe, test, and predict more
abstract phenomena and design systems.
● Develop a model to describe
unobservable mechanisms.
Connections to Nature of Science
Science Models, Laws, Mechanisms, and
Theories Explain Natural Phenomena
Laws are regularities or mathematical
descriptions of natural phenomena.
Scientific Knowledge is Based on
Empirical Evidence
Science knowledge is based upon logical
and conceptual connections between
evidence and explanations.
ETS1.C: Optimizing the Design Solution
The iterative process of testing the most
promising solutions and modifying what is
proposed on the basis of the test results
leads to greater refinement and ultimately to
an optimal solution. One design may not
perform best across all tests. Identify the
characteristics of the design that performed
best in each test case. This information can
be useful for the redesign process.
PS1.B: Chemical Reactions
Substances react chemically in characteristic
ways. In a chemical process, the atoms that
make up the original substances are
regrouped into different molecules, and
these new substances have different
properties from those of the reactants.
The total number of each type of atom is
Systems and System Models
Models can be used to represent systems
and their interactions – such as inputs,
processes, and outputs – and energy and
matter flows within systems.
Energy and Matter Matter is conserved
because atoms are conserved in physical
and chemical processes.
Patterns Macroscopic patterns are related
to the nature of microscopic and atomic-level
structure.
29
conserved, and thus the mass does not
change.
HĀ outcomes practiced: 1. Belonging - students will work in teams, actively participate, and practice communicating with clarity and
confidence. 2. Responsibility - students will be active learners, encouraged to ask for help, will need to set goals and complete tasks with
their team. 4. Aloha - students should learn to appreciate the gifts and talents of their team members, make everyone feel welcomed and
heard, be respectful of all ideas, and share responsibilities.
Before the Activity
● Break up into teams
● Gather the following materials:
Materials List - For this activity, each group will need:
● Vials
● Clock Base
● Black Sticky Foam
● Black Double-Stick Foam
● Beaker
● Scoop
● Stir Stick
● Sandpaper
● LED Clock
● Voltmeter
● Alligator-clip wire
● Sodium Chloride (salt)
● Copper Strips
● Zinc Strips
● Warm Water (not included)
Potential Safety Issues
● None
Potential Obstacles
● Just like in Lab 3, students will need to scratch off the shiny surface of the copper and zinc strips. This
may take a little time.
● It is important to read each step very carefully and to take your time to make sure everything is oriented
in the proper direction, order, and are lined up correctly.
Procedure
1. You already know what to do - lay down your mess mat
2. Follow Steps 1 -18 in the KiwiCo Manual (pages 44 - 50)
Embedded Activity Assessment
30

1. Grab the voltmeter and pick one of the vials to test. Clip the black wire to the zinc strip and the
red wire to the copper strip. Is the voltmeter reading close to 0.75V ? ____________________
If not, take some time to troubleshoot your configuration. Are the wires securely clipped to the
strips? Are the strips touching each other? Review Steps 9-16 if needed to make sure each
step was done correctly.
3. Follow Steps 19 - 24 in the KiwiCo Manual (pages 50 - 52)
2. Did your clock turn on? ____________________ If not, troubleshoot your design. If so,
CONGRATULATIONS! You made electricity with chemistry!
Post-Activity Assessment:
1. How many attempts did it take you to get your clock to work?__________________________
2. Reflection: What lessons did you learn along the way? _______________________________
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
Draw a picture or model below that illustrates what electrons are doing in your saltwater powered
clock.
31
Optional Extension Activity: You can use other items in place of the saltwater solution. Grab a
lemon, orange, even a potato, and stick the two different strips into them. Just like in the saltwater
solution, do not let the metal strips touch. Clip the voltmeter to the strips. How does the voltage of the
item you chose compare to the saltwater battery?
Key Concepts: Engineers use science, math, technology and creativity to solve problems. When it
comes to developing complex electrical systems, it may take many iterations to be successful. In this
activity, you had a guide to help you through the engineering design process. Where you built your
batteries, tested them, and made configuration revisions as needed to create a working clock. Way to
make the best of the pieces (constraints) you had to work with!
Common Misconceptions/Career Connection: Many students are afraid to become engineers
because they do not like math. In this exercise, you were able to practice engineering with very little
mathematics. Math is an important part of engineering, but you do not have to be a mathematician to
pass the required math classes. You just have to get through them, and then you can still be a
successful engineer. The key is grit and perseverance.
32
Renewable Energy Connections: Hydroelectricity from mechanical energy, thermal conversion
energy, and chemical energy all all free, clean and renewable. Scientists and engineers around the
world are researching ways we can improve our technologies to harness “Blue Energy” to help supply
our electricity needs. In the future, we will continue to need scientific and engineering breakthroughs
in the energy sector, and maybe someone, possibly you, will help.
Conclusion
Renewable energy resources are ultimately our best option to continue to power our homes, schools,
and community energy needs. Using renewable energy will help us to reduce our environmental
impact so that we can enjoy the natural gifts and beauty of our planet for generations to come.
The Hawaiʻi State Energy Office is working within its means to set clean energy goals for our state.
However, each of us, as individuals, need to consider our energy consumption habits and create an
action plan for conserving energy. No single person or technology is going to solve our energy needs
or environmental problems. We must all work together to reduce our energy consumption, and to
work toward powering our energy needs with a balanced portfolio of renewable energy resources.
Note that while renewable energy such as hydroelectricity, solar and wind power are often referred to
as “clean energy”, the raw materials needed to create batteries, solar panels, windmill hardware may
come from nonrenewable resources. These include materials like lithium for batteries and metals for
solar cells and wires. This means that even if we successfully transition to 100% renewable energy
for electricity production in our state, it is still important to conserve energy where we can to reduce
the rate of our raw material extraction.
Mahalo nui loa for joining us on this energy exploration into the world of hydroelectricity,
electrochemistry, engineering, and renewable energy resources. With your help, our future will be
bright!
Learn more about Hawai’i’s Clean Energy Initiative by visiting: | Securing the Renewable Future
(Hawaiʻi .gov) energy.Hawaiʻi .gov
Hawaiʻi - QUICK FACTS
■ For more information on Hawaiʻi ’s energy sector and progress in the areas of energy
efficiency, renewable energy, and clean transportation, download the 2020 Hawaiʻi ’s
Energy Facts & Figures (PDF).
■ Hawaiʻi was the first state to set a deadline for generating 100% of its electricity from
renewable energy sources, which is required to be achieved by 2045.
■ Despite being among the five states with the lowest total energy consumption, Hawaiʻi
uses about 11 times more energy than it produces. More than four-fifths of Hawaiʻi 's
energy consumption is petroleum, making it the most petroleum-dependent state.
33

■ In 2019, solar power provided more than half of Hawaiʻi 's total renewable electricity
generation, primarily from small-scale, customer-sited solar panel systems, which have
roughly tripled in capacity since 2015.
■ The amount of Hawaiʻi 's coal-fired generation in 2019 was the lowest since 1992, and coal
fueled 12% of the state’s electricity generation. The state's one coal-fired power plant is
scheduled to be retired in 2022.
■ Hawaiʻi has the highest average electricity retail price of any state, in part because it relies
on petroleum for more than 60% of its electricity generation.
■ Current Renewable portfolio standards (RPS)
○ Hawaiʻi Island - 43%
○ Kauai - 56%
○ Maui County (including Molokai and Lanai) - 41%
○ Oahu - 25%
■ Last Updated: January 21, 2021 SOURCE: EIA.GOV
Words to know:
● Atom: the smallest particle that makes up all matter.
● Batteries: used to store energy.
● Chemical Energy: energy that can be harnessed from chemical reactions like the combustion
of fossil fuels, metabolism when you eat food, or batteries.
● Circuit: a conductive pathway that electricity can flow through.
● Conserve: to use less of something like electricity or water.
● Constraints: The limitations placed on a possible engineering solution.
● Consumption: the use of resources like energy, water, food, minerals, and more.
● Data: facts about something that have been measured, observed and can be analyzed.
● Iterative Design: As you create something, continue to think about new ideas and ways you
can make improvements.
● Engineering: the use of science and math in the design and construction of things.
● Energy: the ability to change things or do work.
● Electrical Engineer: Engineers who use their knowledge of electrical currents and electricity to
develop solutions and designs for electrical systems like buildings, computers, robots, and
even electric cars.
● Electricity: flow of electrical power or charge.
● Fossil Fuels: coal, oil, natural gas. These energy resources come from the fossils of plants and
tiny animals that lived millions of years ago.
● Generator: a device that converts mechanical energy to electrical energy by turning a magnet
inside a coil of wire.
● Hydropower: energy that comes from the force of moving water
● Innovation: a new invention or way of doing something
● Kinetic Energy: the energy of something in motion
● Nonrenewable: an energy source that is not able to be replenished as quickly as it is used.
● Potential Energy: energy waiting to make something happen like water stored in a dam.
● Reclamation: The process of returning land to its original condition or better.
34

● Renewable Energy: a form of energy that doesn’t get used up, including the energy from the
sun, wind, and tides.
● Research: The process of gathering reliable, relevant information and ideas related to a
scientific or technological issue.
● Solar Power: energy from the sun that is converted into electricity.
● Technology: tools, methods, and systems used to solve a problem or do work.
● Tidal Turbines: Imagine a windmill deep underwater. The turbines spin from the changing
directions of waterflow instead of airflow.
● Troubleshoot: as you design a blueprint or build a model, something may not work. In this case
you will need to think about what went wrong and how you can fix it. Sometimes this will be a
process of trial, error, and discovery.
● Turbine: a device that uses pressure on blades by water, air, or steam to spin generators that
create electricity.
● Voltage: Electrical pressure that makes electrons move on or off an atom giving them an
electrical charge.
● Volts: a unit of electrical potential also known as electromotive force.
● Water Cycle: the movement of water from land to bodies of water, into the atmosphere, and
back to earth.
At Home Extension Lessons: (Homework)
35

1. Electricity Reduction Experiment
This week, your child learned all about hydropower, chemistry, electricity, and electrochemistry. They
had the opportunity to explore what components in their school use electricity; different sources of
energy from water; how electricity is produced; how circuits work through hands-on activities; and
they even engineered (designed, built, and tested) their very own battery used to power a LED, and a
saltwater powered clock
They also learned that Hawaiʻi was the first state to set a deadline for generating 100% of its
electricity from renewable energy sources. Our state has a goal to achieve this milestone by 2045. To
achieve this, everyone must get onboard. Take a minute to discuss the following questions with your
child:
1. How do you and your household use electricity in your daily life? _______________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
(You may want to make a list of items in your home that use electricity. Some big consumers are hot
water heaters, refrigerators, washing and drying machines, and other large appliances.)
2. What are some ways that you can reduce your energy consumption? Come up with at least
three action items.
36

3. How will reducing your energy consumption affect our natural resources? ________________
___________________________________________________________________________
___________________________________________________________________________
4. How will reducing your energy affect your family’s finances? To test this, take a look at your
electric bill for this month and then compare 1-month later after practicing your energy pledge
action items.
Original Bill KWH/Day_______________ Conservation Month Bill KWH/Day _______________
Amount Saved? $_______________
To get a better understanding of your energy consumption, you can check your electrical bill. Sit down
as an ʻohana, and look over your electric bill together. It should look something like this:
37
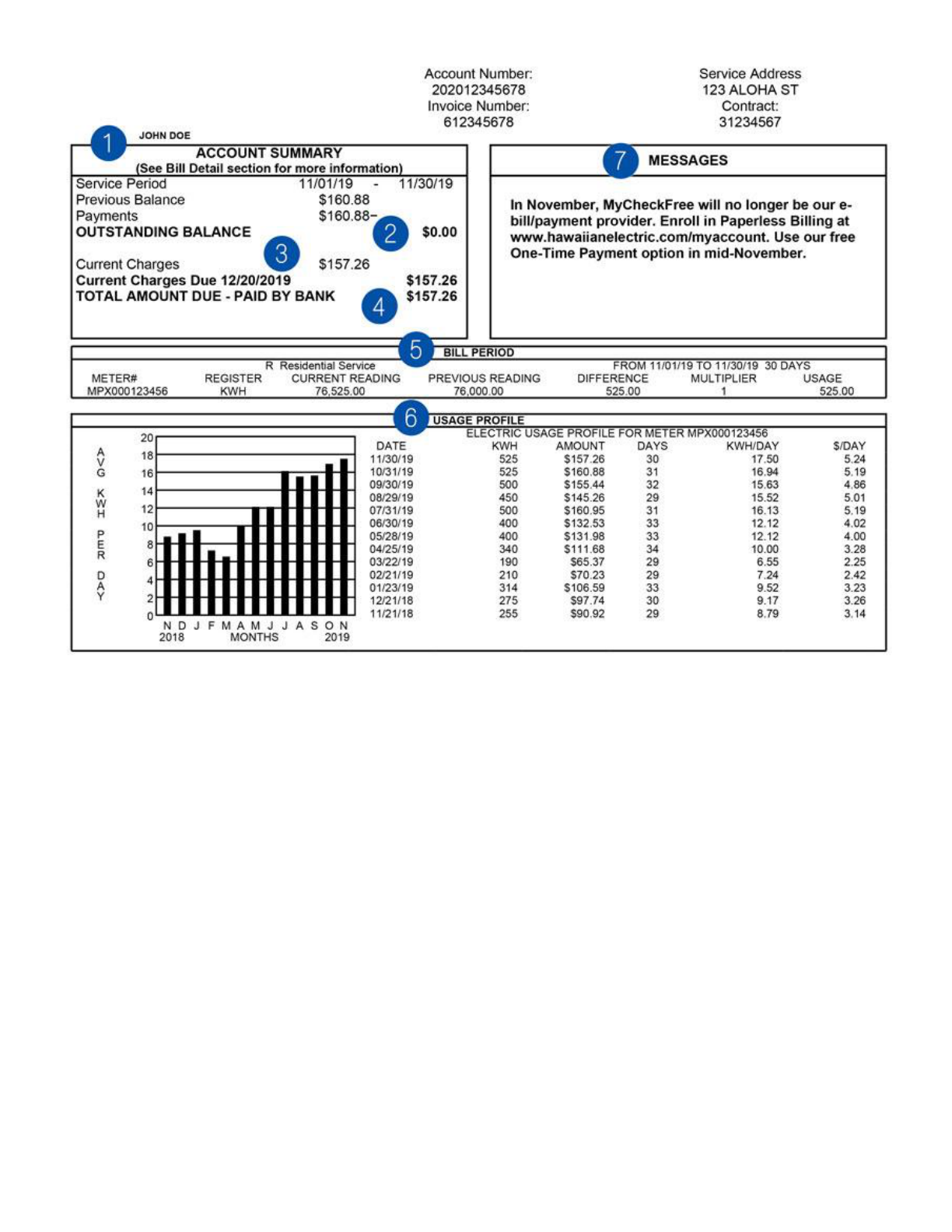
1. Account Summary: This section provides the electric service billing period for the current
bill and summarizes what is owed on the current bill.
2. Outstanding Balance: The previous balance line item shows the total charges on your last
electric bill. The payments you made toward the last bill are subtracted from the previous
balance to determine how much, if anything, remains to be paid toward the previous bill,
that amount is the outstanding balance.
3. Due Date: This is when your payment should be received to avoid a late payment charge.
4. Total Amount Due: This is how much you currently owe. The total amount due includes the
current charges, any adjustments made to your bill, plus the outstanding balance.
Adjustments may include items such as: fees for service establishment, reconnection, late
payment, returned check, or Sun Power for Schools donation.
5. Bill Period: This box contains data that describes your electricity use during the billing
period and the rate schedule (such as R Residential Service) used to compute your electricity
charges. The beginning and ending dates of the electric service billing period and the
number of days in the billing period are provided.
Meter # is the identification number on the electric meter. Register provides the
meter's unit of measure. KWH means kilowatt-hours. For accounts that have two electric
meters, the second meter number and corresponding data will be shown below the data
38

provided for the first meter.
Current Reading is the cumulative number of kilowatt-hours shown on the meter
when it was read for the current electric bill. Previous reading is the cumulative number of
kilowatt-hours shown on the meter when it was read for the previous bill. The difference is
computed by subtracting the previous reading from the current reading. For accounts that
use large amounts of electricity, the meters may not register electricity use by single
kilowatt-hours. They may register electricity use by tens or hundreds of kilowatt-hours. That
is explained by the multiplier. For most residences the multiplier is 1. For large power
users, like a university or hospital, the multiplier may be as high as 240. When the difference
is multiplied by the multiplier, the electricity usage for the billing period is determined in
kilowatt-hours. At times, your electric bill may have to be estimated. In those cases, (EST) will
be printed on the bill next to the current reading.
Residential and Schedule G commercial customers with advanced meters may see a
KW line item underneath their KWH usage. Please disregard this at this time as it does not
factor into your bill calculation. It may be used in the future when additional rate options and
programs become available.
6. Usage Profile: This section provides you with a historical view of your electricity use. The
handy bar graph on the left side tells you at a glance how much your average daily electricity
use has fluctuated over the past year. The electric usage profile for your meter can help you
monitor your electricity use. It provides a record of the electricity use for your account for
the past year. The date is the ending date of a billing period. KWH is the number of
kilowatt-hours used during that period. The amount and days are the total current charges
on your electric bill and the number of days in that billing period, respectively. KWH/day
lists the average number of kilowatt-hours of electricity used per day during the period.
$/day tells you, on average, how much your electricity costs per day.
7. Messages: This area contains useful information and tips for managing your electricity use.
It also may contain specific messages for individual customers about their electric account.
2. Cookie Mining & Reclamation Experiment
39

Things that people use from the natural world around us, like plants, minerals, water, wind, and
sunshine, are called natural resources. Natural resources help to sustain populations by providing
humans with food, shelter, technologies, and energy.
In this activity, you and your child will explore how taking resources from nature through a process
called mining can negatively affect the natural environment. A few things that we mine (or take) from
the earth include:
● Fossil Fuels like Coal and Petroleum (we burn for electricity generation)
● Copper (used for wires and electrical hardware)
● Rare-Earth Minerals (for electronics)
● Steel & Iron (used for wind turbines)
● Lithium (used in batteries)
Let’s test for ourselves what impact mining can have on the ʻāina.
Materials:
For this activity, you will need:
● 1 chocolate chip cookie (or more if it is snack time)
● 1 toothpick or “mining tool” of some sort. Be careful with sharp objects.
● A mess mat or hard surface (place mat, wax paper, something that won’t shred easily).
● Graph or regular paper
● Pencil or pen
● Timer (optional)
Procedure:
1. Gather your materials, and place your cookie on the graph paper.
2. Trace around the cookie with a pencil.
3. Using only the toothpick as a mining tool, extract the chocolate chip
“coal” from the cookie. Do not use your fingers.
4. Place each piece of extracted “coal” outside of your mining area.
5. Set the timer for two minutes. When the time is up, count your coal.
6. Look back at your original cookie outline. Now you need to reclaim your
mined area by putting the cookie back together.
Discussion:
1. Were you able to put the land (cookie) back together?
2. Knowing that you will want to reclaim the land, does it make sense to extract all the “coal”?
Reclamation is a process that mining companies use to try to return the land back to the way it was
to prevent habitat loss, erosion, and to restore ecological balance. However, as you saw here in this
experiment, reclamation can sometimes take a long time and can be difficult to do.
40

EXTERNAL RESOURCES ON PERFORMANCE EXPECTATIONS
How to Read the Next Generation Science Standards
NGSS Webpage that allows you to search for Performance Expectations based on grade, discipline,
SEP, DCI, and/or CCC
Evidence Statements (describe a detailed look at the NGSS performance expectations)
NSTA Performance Expectation Finder
41

